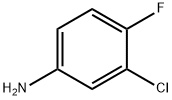
Benzenemethanamine, N-(3-chloro-4-fluorophenyl)-3,4-dimethoxy- synthesis
- Product Name:Benzenemethanamine, N-(3-chloro-4-fluorophenyl)-3,4-dimethoxy-
- CAS Number:416865-75-3
- Molecular formula:C15H15ClFNO2
- Molecular Weight:295.74
Yield:416865-75-3 94%
Reaction Conditions:
with sodium cyanoborohydride;acetic acid in isopropyl alcohol at 1 - 20; for 3 - 4 h;
Steps:
1.B
[0131] Procedure B. A mixture of 3-chloro-4-fluoroaniline (75.0 g, 515 mmol), 3,4-dimethoxybenzaldehyde (85.6 g, 515 mmol) and isopropyl alcohol (755 mL) was stirred until a homogeneous solution was obtained. After cooling to -1° C. +/-2° C., acetic acid (31.1 g, 518 mmol) was added to the reaction mixture followed by sodium cyanoborohydride (38.9 g, 619 mmol). The reaction mixture was stirred at ambient temperature until completion of the reaction (3-4 h). The reaction was quenched with 1 N NaOH (aq) (515 mL) and the resulting slurry cooled to 0° C., held for 20-30 min, then filtered and washed with water until the pH of the product cake was neutral. The product cake was dried in a vacuum oven at 50° C. to yield (3-chloro-4-fluoro-phenyl)-(3,4-dimethoxy-benzyl)-amine (143.3 g, 94%).
References:
US2004/158065,2004,A1 Location in patent:Page 17-18
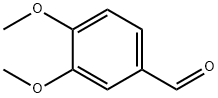
120-14-9
753 suppliers
$5.00/25g

416865-75-3
3 suppliers
inquiry