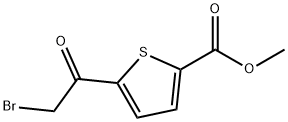
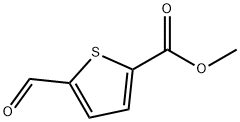
5-(2-BroMo-acetyl)-thiophene-2-carboxylic acid Methyl ester synthesis
- Product Name:5-(2-BroMo-acetyl)-thiophene-2-carboxylic acid Methyl ester
- CAS Number:4192-32-9
- Molecular formula:C8H7BrO3S
- Molecular Weight:263.11
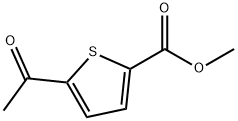
4101-81-9
34 suppliers
$129.00/1g

4192-32-9
19 suppliers
$250.00/0.25G
Yield:4192-32-9 69%
Reaction Conditions:
with copper(ll) bromide in ethyl acetate; for 6 h;Heating / reflux;
Steps:
5
A mixture of 1.7 g (lOmmol) of 5-acetyl-thiophene-2-carboxylic acid, 0. 75 mL MeI (12 mmol), 4.3 g of Na2C03 (35mmol) in DMF 10 mL was allowed to stir at room temperature overnight. Quench with water with some addition of sat. NH4Cl sol. Precipitate formed and was collected to yield 1.48g of 5-acetyl-thiophene-2-carboxylic acid methyl ester as a white solid. Yield 80%. A mixture of 5.1 g of 5-acetyl-thiophene-2-carboxylic acid methyl ester, 11 g of CuBr2 in 50 mL of EtOAc was refluxed for 6 hrs. The reaction mixture was cooled to rt and filtered through diatomaceous earth and purified by combiflash to yield 5 g of 5- (2- bromo-acetyl) -thiophene-2-carboxylic acid methyl ester.. Yield 69%. A mixture of 263 mg (lmmol) of the above ester and 0.4 mL of formamide was heated to 120°C. Then a mixture of 0.2 mL and 0.1 g of concentrated H2S04 was added. The reaction mixture was heated at 120°C for 1 h. The reaciton mixture was cooled down to room temperature and poured into water, and extracted with ether. The ethereal extract was dried over MgS04, and concentrated in vacuo. The residue was purified by combiflash to yield 60 mg of 5-oxazol-4-yl-thiophene-2-carboxylic acid methyl ester as light yellow solid. Yield 30%. The above ester was dissolved in 3 mL THF, 1 mL MeOH and 1 mL 1N LiOH solution and stirred overnight. The solvent was removed. Acetic acid was added and a light yellow precipitate formed which was collected by filtration to yield 20 mg of the title compound. Yield 33%.
References:
WO2005/79791,2005,A1 Location in patent:Page/Page column 126-127
4066-41-5
210 suppliers
$6.00/1g

4192-32-9
19 suppliers
$250.00/0.25G
4565-31-5
147 suppliers
$19.00/250mg

4192-32-9
19 suppliers
$250.00/0.25G
67808-64-4
114 suppliers
$19.00/250mg

4192-32-9
19 suppliers
$250.00/0.25G

50340-79-9
90 suppliers
$24.00/250mg

4192-32-9
19 suppliers
$250.00/0.25G