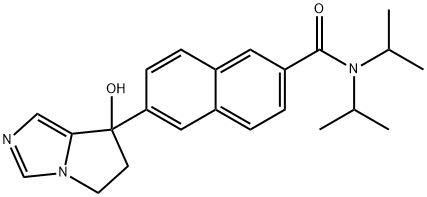
6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide synthesis
- Product Name:6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide
- CAS Number:426219-32-1
- Molecular formula:C18H17N3O2
- Molecular Weight:307.3465
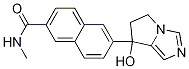
![2-Naphthalenecarboxamide, 6-[1,3-dihydroxy-1-[1-(triphenylmethyl)-1H-imidazol-4-yl]propyl]-N-methyl-](/CAS/20210305/GIF/566200-77-9.gif)
566200-77-9
0 suppliers
inquiry
![6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide](/CAS/GIF/426219-32-1.gif)
426219-32-1
3 suppliers
inquiry
Yield:426219-32-1 80%
Reaction Conditions:
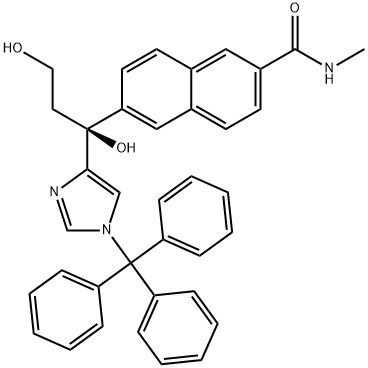
Stage #1: 6-[1,3-dihydroxy-1-(1-trityl-1H-imidazol-4-yl)propyl]-N-methyl-2-naphthamidewith methanesulfonyl chloride;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;water;dimethyl sulfoxide at 0 - 8; for 1.5 h;
Stage #2: with N-ethyl-N,N-diisopropylamine in methanol;acetonitrile at 60 - 63; for 2.33333 h;
Stage #3: with ammonium chloride in methanol;water;ethyl acetate;acetonitrile at 25;
Steps:
3 Example 3: Preparation of 6-[7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide
20 ML of THF and 1.23 ML (3.14 mmol, 2eq) of diisopropylethylamine were added to 2 g (3.523 mmol) of 6-[1,3-dihydroxy-1-(1-trityl-1H-imidazol-4-yl)propyl]-N-methyl-2-naphtamide. 20 ML of THF was further added. 0.35 ML (4.58 mmol, 1.3eq) of methylsulfonyl chloride was added dropwise at 2~3°C, and the mixture was stirred at 2~3°C for 25 minutes. 16 ML of dimethyl sulfoxide was added dropwise at 2~3°C, and the mixture was stirred at 0~3°C for 45 minutes. 0.2 ML of methylsulfonyl chloride and 0.5 ML of diisopropylethylamine were added at 0~3°C, and the mixture was stirred at 0~3°C for 20 minutes. 4 ML of water was added dropwise at 0~8°C, and the layers were separated.. The aqueous layer was re-extracted with 10 ML of ethyl acetate two times, the organic layers were combined, and washed with 4 ML of an aqueous saturated sodium chloride solution two times.. The material was dried with magnesium sulfate, and concentrated under reduced pressure.. The concentration residue was dissolved in 15 ML of acetonitrile, and the solution was stirred at 60~63°C for 20 minutes.. To the reaction solution were added 4.5 ML of methanol and 1.23 ML (3.14 mmol, 2eq) of diisopropylethylamine.. The mixture was stirred at 60~63°C for 2 hours.. After cooled to 25°C, 30 ML of an aqueous saturated ammonium chloride solution and 40 ML of ethyl acetate were added, and the layers were separated.. The organic layer was back extracted with 10 ML of 0.5N hydrochloric acid-aqueous saturated ammonium chloride solution.. The aqueous layers were combined, a PH was adjusted to 8 with a 30% aqueous sodium hydroxide solution, followed by stirring at 25°C for 18 hours and 15 minutes, and at 0~5°C for 1 hour and 25 minutes.. Crystals were filtered, and washed with water.. Vacuum drying (50°C) to a constant weight afforded 0.87 g of 6-[7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide (yield 80%).1H NMR ((CDCl3+CD3OD): δ 2.89-3.02 (2H, m), 3.04 (3H, d,J=4.6 Hz), 4.12-4.25 (1H, m), 4.27-4.43 (1H, m), 6.79 (1H, s), 7.20 (1H, q, J=4.6 Hz), 7.54 (1H, s), 7.63 (1H, dd,J=8.6, 1.8 Hz), 7.83 (2H, s),7.89 (1H, d, J=8.6 Hz), 8.03 (1H, s), 8.28 (1H, s).
References:
EP1471056,2004,A1 Location in patent:Page 33-34
566200-79-1
9 suppliers
inquiry
![6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide](/CAS/GIF/426219-32-1.gif)
426219-32-1
3 suppliers
inquiry
426219-35-4
77 suppliers
$90.00/100mg
![7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI)](/CAS/20150408/GIF/426219-43-4.gif)
426219-43-4
42 suppliers
$65.00/10mg
![6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide](/CAS/GIF/426219-32-1.gif)
426219-32-1
3 suppliers
inquiry
![7H-Pyrrolo[1,2-c]imidazol-7-one,5,6-dihydro-(9CI)](/CAS/20150408/GIF/426219-43-4.gif)
426219-43-4
42 suppliers
$65.00/10mg
![6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide](/CAS/GIF/426219-32-1.gif)
426219-32-1
3 suppliers
inquiry