
4-(2-CHLORO-ACETYL)-3,4-DIHYDRO-1H-QUINOXALIN-2-ONE synthesis
- Product Name:4-(2-CHLORO-ACETYL)-3,4-DIHYDRO-1H-QUINOXALIN-2-ONE
- CAS Number:436088-67-4
- Molecular formula:C10H9ClN2O2
- Molecular Weight:224.64
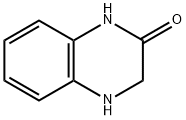
59564-59-9
143 suppliers
$30.00/250mg

79-04-9
373 suppliers
$12.00/5g

436088-67-4
26 suppliers
$85.00/250mg
Yield:436088-67-4 95%
Reaction Conditions:
Stage #1: 1,2,3,4-tetrahydroquinoxalin-2-one;chloroacetyl chloridewith triethylamine in ethyl acetate; for 1 h;Reflux;
Stage #2: with potassium carbonate in ethyl acetate; for 0.25 h;
Steps:
4.3.3 General method for the preparation of N-acylated tQNXs (1-16)
The tQNX1-4 stored as chlorhydrate salts were converted to their free bases as described above. In a typical acylation reaction, the corresponding acylating agent (1.2mmol) was added to a solution of tQNX (1mmol) in EtAcO (30ml) and triethylamine (1.2mmol) and heated to reflux for 1h. After cooling, 50ml of a 10% solution of K2CO3 was added and the mixture was stirred for additional 15min. The solution was left still, and the organic phase was separated. The organic phase was then sequentially washed with HCl 0.5M, 10% K2CO3 and water, dried with Na2SO4 and concentrated. The product was characterized without further purification.
References:
Estrin, Darío;Fabian, Lucas;Gómez, Natalia;Moglioni, Albertina;Salvatori, Melina;Taverna Porro, Marisa;Turk, Gabriela [European Journal of Medicinal Chemistry,2020,vol. 188]
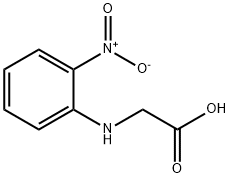
5427-99-6
9 suppliers
inquiry

436088-67-4
26 suppliers
$85.00/250mg

1493-27-2
524 suppliers
$5.00/10g

436088-67-4
26 suppliers
$85.00/250mg
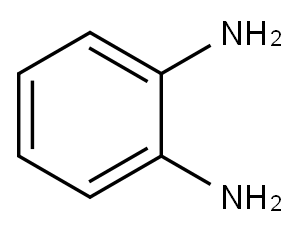
95-54-5
536 suppliers
$9.00/1g

436088-67-4
26 suppliers
$85.00/250mg