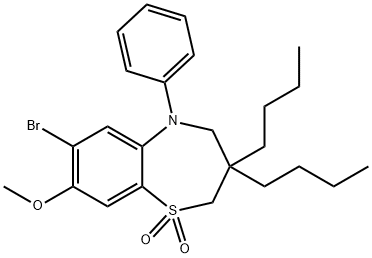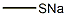
1,5-Benzothiazepin-8-ol, 3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-5-phenyl-, 1,1-dioxide synthesis
- Product Name:1,5-Benzothiazepin-8-ol, 3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-5-phenyl-, 1,1-dioxide
- CAS Number:439088-16-1
- Molecular formula:C24H33NO3S2
- Molecular Weight:447.65
Yield:439088-16-1 84%
Reaction Conditions:
with sodium tetrahydroborate in N,N-dimethyl-formamide at 60; for 15 h;
Steps:
1.3 Preparation of 3,3-dibutyl-8-hydroxy-7-methylthio-5-phenyl-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepine dioxide
7-Bromo-3,3-dibutyl-8-methoxy-2,3-dihydrobenzo[b][1,4]thiazepine-4(5H)-one (5.77 g, 14.44 mmol) obtained in Step 2) was charged with iodobenzene (50 mL), copper iodide (0.55 g, 2.89 mmol), potassium carbonate (4 g, 28.9 mmol), and tris[2-(2-methoxyethoxy)ethyl]amine (0.5 mL, 1.44 mmol), stirred at room temperature for about 5 minutes, and refluxed at 190°C for 17 hours. The resultant was cooled to room temperature, filtered with silica, and washed with hexane to remove iodobenzene. The silica-captured compound was eluted with ethyl acetate and dichloromethane, and the recovered solution was concentrated to obtain 5.8 g of 7-bromo-3,3-dibutyl-8-methoxy-5-phenyl-2,3-dihydrobenzo[b][1,4]thiazepine-4(5H)-one. Lithium aluminum hydride (1.4 g, 36.88 mmol) was added to a 500 mL round-bottom flask and dried under vacuum. Diethyl ether (150 mL) was added thereto, cooled to -10°C, and then anhydrous sulfate (1 mL, 18.44 mmol) was added slowly dropwise thereto. The thus-obtained 7-bromo-3,3-dibutyl-8-methoxy-5-phenyl-2,3-dihydrobenzo[b][1,4]thiazepine-4(5H)-one was dissolved in diethyl ether and added dropwise to the reaction flask. The resultant was stirred at room temperature for 2 hours, and charged with a saturated ammonium chloride solution to extract the diethyl ether layer. The extract was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 5 g of 7-bromo-3,3-dibutyl-8-methoxy-5-phenyl-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepine. The thus-obtained compound (5 g, 10.98 mmol) was charged with 75 mL of a mixed solvent (tetrahydrofuran : t-butanol = 1 : 1), osmium tetroxide (0.07 g, 0.27 mmol), and N-methylmorpholine N-oxide (3.96 g, 32.94 mmol), and stirred at room temperature for 12 hours. A saturated ammonium chloride solution and dichloromethane were added thereto to extract the dichloromethane layer, and the extract was concentrated to obtain 3.55 g of 7-bromo-3,3-dibutyl-8-methoxy-5-phenyl-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepine dioxide as an ivory solid. The thus-obtained compound (3.55 g, 7.18 mmol) was charged with dimethylformamide (100 mL), sodium thiomethoxide (5.032 g, 71.79 mmol), and sodium borohydride (5.43 g, 143.6 mmol), and stirred at 60°C for 15 hours. A saturated ammonium chloride solution and t-butyl methyl ether were added thereto to extract the ether layer, and this was concentrated. The concentrated compound was charged with hexane, and the solid was filtered to obtain 2.7 g of 3,3-dibutyl-8-hydroxy-7-methylthio-5-phenyl-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepine dioxide, with 84% yield. 1H NMR (400 MHz, CDCl3) δ 7.31 (s, 1H), 7.16 (t, J = 8 Hz, 2H), 6.92 (d, J = 8 Hz, 2H), 6.84 (t, J = 6.8 Hz, 1H), 6.67 (s, 1H), 3.61 (s, 2H), 3.07 (s, 2H), 2.15 (s, 3H), 1.28-1.44 (m, 4H), 1.97-1.19 (m, 8H), 0.74 (t, J = 6.8 Hz).
References:
EP3210977,2017,A2 Location in patent:Paragraph 0049-0051
1747-60-0
401 suppliers
$6.00/25g

439088-16-1
19 suppliers
inquiry
6274-29-9
43 suppliers
$180.00/250mg

439088-16-1
19 suppliers
inquiry

439089-27-7
34 suppliers
inquiry

439088-16-1
19 suppliers
inquiry