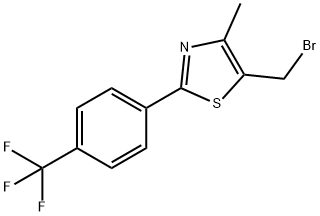
5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE synthesis
- Product Name:5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE
- CAS Number:439134-78-8
- Molecular formula:C12H9BrF3NS
- Molecular Weight:336.17
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
66 suppliers
$65.00/50mg
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
44 suppliers
$72.50/250mg
Yield:439134-78-8 93%
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in dichloromethane at 20; for 1 h;
Steps:
3 Example 3; Preparation [OF 5-BROMOMETHYL-4-METHYL-2- [ (4-TRIFLUOROMETHYL)] phenyl] thiazole [Step C]
[[4-METHYL-2- (4-TRIFLUOROMETHYL-PHENYL) THIAZOLE-5-YL]] methanol (15.0 g, 55.0 mmol) obtained from Example 2 was dissolved in anhydrous dichloromethane [(300] [MNo. ], and then triphenylphosphine (TPP, 5. 7 g, 60.0 mmol, 1.1 eq. ) and tetrabromomethane (20.0 [G,] 60.0 mmol, 1.1 eq. ) were added to the mixture [SEQUENCIALLY] at room temperature. After 1 hour, the solvent was evaporated from the reaction mixture under reduced pressure. Subsequently, the remained triphenylphosphine oxide was precipitated by a mixed solvent of hexane and ethyl acetate (v/v = [5/1),] followed by filtration and evaporation under reduced pressure to thereby yield [17. 2] g of the title compound (yield: 93%). 'H-NMR (300 MHz, [CDC13)] : 8.00 (d, 2H, [J =] 8.1 Hz), 7.67 (d, 2H, [J =] 8.2 Hz), 4.72 (s, 2H), 2.47 (s, 3H). [13C-NMR] (78. [5] MHz, [CDC13)] : 165. 0, [153.] 8, [136. 9, 132. 4,] 129.7 (q), [127.] 0, 126.3 (m), 122.5, 23.8, 15.5.
References:
WO2003/106442,2003,A1 Location in patent:Page 13
72505-21-6
160 suppliers
$10.00/5g
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
44 suppliers
$72.50/250mg
![Ethyl 4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazole-5-carboxylate](/CAS/GIF/175277-03-9.gif)
175277-03-9
120 suppliers
$15.00/5g
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
44 suppliers
$72.50/250mg