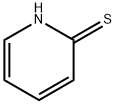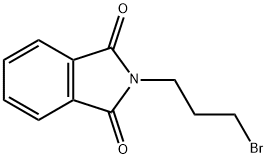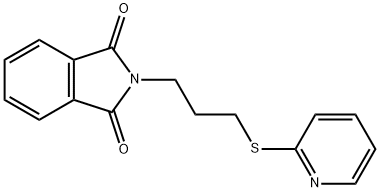
2-[3-(pyridin-2-ylsulfanyl)propyl]-2,3-dihydro-1H-isoindole-1,3-dione synthesis
- Product Name:2-[3-(pyridin-2-ylsulfanyl)propyl]-2,3-dihydro-1H-isoindole-1,3-dione
- CAS Number:443120-58-9
- Molecular formula:C16H14N2O2S
- Molecular Weight:298.36
Yield:443120-58-9 99%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 90; for 6 h;Inert atmosphere;
Steps:
4.2. General procedure for compounds 7a-r
General procerure: To a suspension of mercaptan substituent heterocyclic in DMF, one equivalent of N-(3-bromopropyl) phthalimide and K2CO3 (1 equivalent) were added. The reaction was stirred for 6 h at 90 °C before cooled to room temperature. After evaporation of the DMF under vacuum, the residue was diluted with methylene chloride, washed with water and brine, and dried over MgSO4. After evaporation of the solution, the crude products were purified by column chromatography eluting with 2.5:1 petroleum ether/acetone to afford the desired products as yellow or white solid.
References:
Chen, Xiao-Zhuo;Xu, Peng;Liu, Lu;Zheng, Dan;Lei, Ping-Sheng [European Journal of Medicinal Chemistry,2011,vol. 46,# 1,p. 208 - 217] Location in patent:experimental part