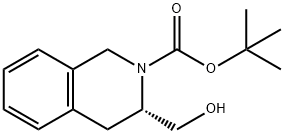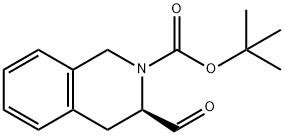
(R)-3-FORMYL-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(R)-3-FORMYL-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:444583-19-1
- Molecular formula:C15H19NO3
- Molecular Weight:261.32
![2(1H)-Isoquinolinecarboxylic acid, 3,4-dihydro-3-[(methoxymethylamino)carbonyl]-, 1,1-dimethylethyl ester, (3R)-](/CAS/20210305/GIF/444583-18-0.gif)
444583-18-0
0 suppliers
inquiry

444583-19-1
13 suppliers
inquiry
Yield:444583-19-1 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether at 0; for 1 h;
Steps:
214.B Step B.; (3R)-tert-Butyl 3-formyl-3,4-dihydroisoquinoline-2(1H)-carboxylate
Lithium aluminum hydride (1 M in THF, 5.3 ml, 5.3 mmol) was added to a solution of (3R)-tert-butyl 3-(methoxy(methyl)carbamoyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (1.7 g, 5.0 mmol theory) in ether (16 ml) stirring at 0° C. under nitrogen. After stirring at 0° C. for 1 h, the reaction was slowly quenched with water (0.40 ml). Sodium hydroxide (1N, 0.80 ml) followed by water (0.60 ml) were then added and the resultant gelatinous precipitate was filtered through Celite rinsing with ether and THF. The filtrate was evaporated and the residue transferred to a separatory funnel with EtOAc. Washing with brine and drying with MgSO4 afforded crude product (1.3 g, 100% yield) which was used in the next step without further purification
References:
US2005/80074,2005,A1 Location in patent:Page/Page column 72

845543-81-9
13 suppliers
inquiry

444583-19-1
13 suppliers
inquiry

24424-99-5
822 suppliers
$13.50/25G

444583-19-1
13 suppliers
inquiry
103733-65-9
253 suppliers
$5.00/100mg

444583-19-1
13 suppliers
inquiry