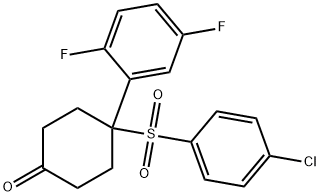
4-(4-chlorophenylsulfonyl)-4-(2,5-difluorophenyl)cyclohexanone synthesis
- Product Name:4-(4-chlorophenylsulfonyl)-4-(2,5-difluorophenyl)cyclohexanone
- CAS Number:471903-20-5
- Molecular formula:C18H15ClF2O3S
- Molecular Weight:384.82

471905-61-0
3 suppliers
inquiry

38053-91-7
136 suppliers
$10.00/250mg

471903-20-5
9 suppliers
inquiry
Yield:471903-20-5 90%
Reaction Conditions:
Stage #1: 2-[1-[(4-chlorophenyl)sulfonyl]ethenyl]-1,4-difluorobenzene;2-trimethylsilyloxy-1,3-butadiene in xylenes at 130;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;xylenes at 50;
Steps:
4 Example 4; 4-[(4-chlorophenyl)sulfonyl]-4-(2,5-difluorophenyl)cyclohexanone
A solution [OF 2- [1- [ (4-CHLOROPHENYL) SULFONYL] ETHENYL]-1,] 4- [DIFLUOROBENZENE] (Example 3) [(100G,] 0. [32MOL)] in xylenes [(500ML)] was azeotropically distilled at [38°C,] 20 mmHg, until 300mL solvent had been removed. 2-Trimethylsilyloxybutadiene (90.4 g, 0. [64MOL)] was then added under a nitrogen atmosphere and the mixture heated to 130 [°C.] After the reaction was completed, the mixture was distilled in vacuo to remove residual diene, whilst maintaining a constant volume by the addition of xylenes (400 mL). The mixture was cooled to [50°C] and THF (500 mL) and 3M [HC1] (424 mL, 1. 27mol) were added. After the hydrolysis was complete, the layers were separated. The organic layer was washed with water (300 mL) and then concentrated by atmospheric distillation until 350 mL of solvent had been removed. The solution was allowed to cool until [CRYSTALLISATION] started, heptane (600mL) was added and the resulting mixture cooled to ambient. The solids were filtered and washed sequentially with heptane: xylenes (3: 1,200 ml) and then heptane [(200ML).] Drying overnight in vacuo at [40°C] furnished [4- [ (4-CHLOROPHENYL) SULFONYL]-4-] (2, 5-difluorophenyl) cyclohexanone (110 g, [90% YIELD). 1H NMR CDC13] 7.43- 7.37 (4H, [M),] 7.22-7. 1 (2H, [M),] 6.97-6. 9 [(1H,] [M),] 3.05-2. 98 (4H, [M)] and 2.61-2. 53 (4H, [M).]
References:
WO2004/13090,2004,A1 Location in patent:Page 12-13
![1-Cyclohexene-1-carboxylic acid,5-[(4-chlorophenyl)sulfonyl]-5-(2,5-difluorophenyl)-2-hydroxy-, Methylester](/CAS/20150408/GIF/471903-19-2.gif)
471903-19-2
1 suppliers
inquiry

471903-20-5
9 suppliers
inquiry
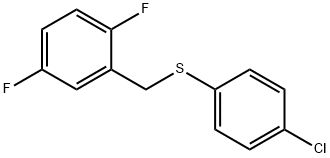
470716-52-0
8 suppliers
$437.00/1g

471903-20-5
9 suppliers
inquiry
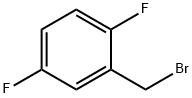
85117-99-3
241 suppliers
$7.00/1g

471903-20-5
9 suppliers
inquiry
![Cyclohexanecarboxylic acid, 5-[(4-chlorophenyl)sulfonyl]-5-(2,5-difluorophenyl)-2-oxo-, methyl ester](/CAS/20210305/GIF/852312-96-0.gif)
852312-96-0
0 suppliers
inquiry

471903-20-5
9 suppliers
inquiry