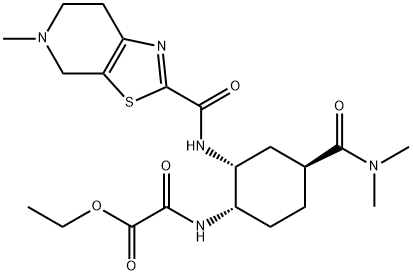
Acetic acid, 2-[[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]amino]-2-oxo-, ethyl ester synthesis
- Product Name:Acetic acid, 2-[[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]amino]-2-oxo-, ethyl ester
- CAS Number:480450-85-9
- Molecular formula:C21H31N5O5S
- Molecular Weight:465.57
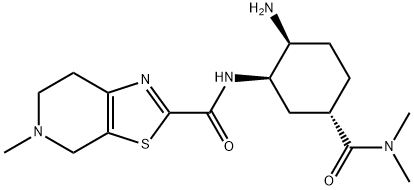
480450-71-3
17 suppliers
inquiry
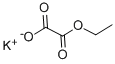
1906-57-6
72 suppliers
$45.00/250mg
![Acetic acid, 2-[[(1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]amino]-2-oxo-, ethyl ester](/CAS/20200611/GIF/480450-85-9.gif)
480450-85-9
23 suppliers
inquiry
Yield:-
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in DMF (N,N-dimethyl-formamide) at 20; for 14 h;
Steps:
269 ethyl 2-[((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)-carbonyl]amino}cyclohexyl)amino]-2-oxoacetate:
[Referential Example 269] ethyl 2-[((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)-carbonyl]amino}cyclohexyl)amino]-2-oxoacetate: The compound (1.5 g) obtained in Referential Example 253 was dissolved in N,N-dimethylformamide (15 ml), and potassium 2-ethoxy-2-oxoacetate (962 mg), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.18 g) and 1-hydroxybenzotriazole monohydrate (227 mg) were added to stir the mixture at room temperature for 14 hours.. After the solvent was distilled off under reduced pressure, a saturated aqueous solution of sodium hydrogencarbonate and methylene chloride were added to the residue to conduct liquid separation.. The resultant organic layer was dried over anhydrous sodium sulfate.. After the solvent was distilled off under reduced pressure, the residue was purified by flash column chromatagraphy on silica gel (methylene chloride:methanol = 47:3) to obtain the title compound (1.13 g).1H-NMR (CDCl3) δ: 1.37(3H,t,J=7.1Hz), 1.55-2.15(6H,m), 2.52(3H,s), 2.77-2.89(3H,m), 2.94(5H,br.s), 3.06(3H,s), 3.71(1H,d,J=15.5Hz), 3.73(1H,d,J=15.5Hz), 4.06-4.13(1H,m), 4.32(2H,q,J=7.1Hz), 4.60-4.63(1H,m), 7.39(1H,d,J=8.3Hz), 7.83(1H,d,J=7.6Hz). MS (ESI) m/z: 466(M+H)+.
References:
EP1405852,2004,A1 Location in patent:Page 186