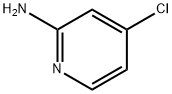
2-Amino-4-chloropyridine synthesis
- Product Name:2-Amino-4-chloropyridine
- CAS Number:19798-80-2
- Molecular formula:C5H5ClN2
- Molecular Weight:128.56
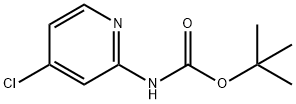
130721-78-7
126 suppliers
$9.00/100mg

19798-80-2
534 suppliers
$6.00/1g
Yield:19798-80-2 100%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 18 h;
Steps:
32 Synthesis of 4-Chloropyridin-2-amine (compound 65-2)
Synthesis of 4-Chloropyridin-2-amine (compound 65-2)[0000228] To a stirred solution of tert-butyl 4-chloropyridin-2-ylcarbamate (2 g, 8.7 mmol, 1 equiv) was addeda 4N solution of hydrogen chloride in dioxane (10 mL, 40 mmol,4.6 equiv) . The solution was allowed to stir for 18 hours atroom temperature. The reaction was concentrated under reduced pressure to give compound 65-2 (HC1) as a reddish yellow solid (1.5 g, 100% yield)
References:
WO2013/123215,2013,A2 Location in patent:Paragraph 0000228
99586-65-9
234 suppliers
$14.00/5g

19798-80-2
534 suppliers
$6.00/1g
942947-94-6
187 suppliers
$22.00/1g

73183-34-3
563 suppliers
$6.00/5g

19798-80-2
534 suppliers
$6.00/1g
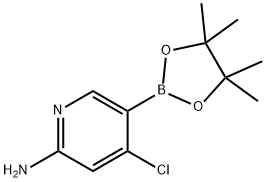
944401-60-9
45 suppliers
$118.00/250mg
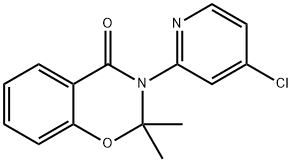
74405-00-8
4 suppliers
inquiry

19798-80-2
534 suppliers
$6.00/1g
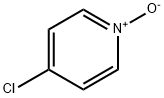
1121-76-2
200 suppliers
$8.00/1g

19798-80-2
534 suppliers
$6.00/1g