
3-CYCLOHEXYL-1H-INDOLE-6-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:3-CYCLOHEXYL-1H-INDOLE-6-CARBOXYLIC ACID METHYL ESTER
- CAS Number:494799-18-7
- Molecular formula:C16H19NO2
- Molecular Weight:257.33
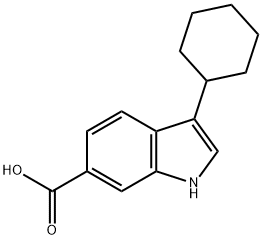
494799-17-6
52 suppliers
$52.50/250mg

67-56-1
739 suppliers
$9.00/25ml

494799-18-7
17 suppliers
inquiry
Yield:494799-18-7 100%
Reaction Conditions:
with thionyl chloride at 0; for 24 h;Heating / reflux;
Steps:
1.2
Step 2; methy 3-cvclohexyl-1H-indole-6-carboxylate; A solution (0.5 M) of 3-cyclohexenyl-1H-indole-6-carboxylic acid (from Step 1) in THF/M eOH (0.5 M, 1:1, v/v) was hydrogenated for 14 h over Pd(OH)2/C (0.1 eq., 20%) at 60 psi. The catalyst was removed by filtration on a pad of celite and the filtrate evaporated to dryness to afford 3-cyclohexyl-1 H-indoIe-6-carboxylic acid (90%) as a white solid 1H NMR (300 MHz, DMSO-d6, 300 K) 6 1.20 -1.53 (m, SH), L70-L87 (m, 3H), 1.90-2.02 (m, 2H), 2.69-2.86 (m, 1H), 7.40 (s, 1H), 7.55-7.65 (rn, 2H), 8.0 (s, 1H), 11.40 (s, 1H); MS (ES+ m/z 244 (M+H)+. A solution of the foregoing compound in MeOH {0.4 M) was treated at 0 °C with thionylchloride (0.5 eq.) and refluxed for 24 h. Volatiles were removed under vacuum to afford the title compound (100%) as a solid.
References:
WO2006/29912,2006,A1 Location in patent:Page/Page column 21-22

494799-17-6
52 suppliers
$52.50/250mg

74-88-4
341 suppliers
$15.00/10g

494799-18-7
17 suppliers
inquiry
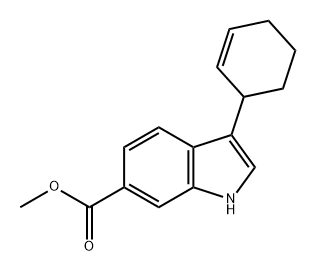
774213-78-4
0 suppliers
inquiry

494799-18-7
17 suppliers
inquiry

494799-17-6
52 suppliers
$52.50/250mg

494799-18-7
17 suppliers
inquiry
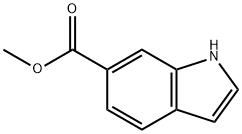
50820-65-0
239 suppliers
$6.00/1g

108-94-1
531 suppliers
$12.00/50g

494799-18-7
17 suppliers
inquiry