
5-(2-FURYL)-1-PHENYL-1H-PYRAZOLE-3-CARBOXYLIC ACID synthesis
- Product Name:5-(2-FURYL)-1-PHENYL-1H-PYRAZOLE-3-CARBOXYLIC ACID
- CAS Number:100537-55-1
- Molecular formula:C14H10N2O3
- Molecular Weight:254.24
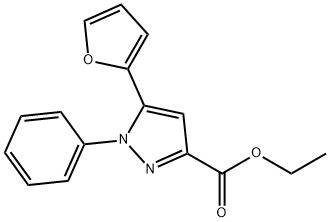
101351-11-5
1 suppliers
inquiry

100537-55-1
19 suppliers
$203.00/500mg
Yield:-
Reaction Conditions:
with sodium hydroxide in methanol;water;Inert atmosphere;
Steps:
General procedure for synthesis of compounds A12, A13
General procedure: A mixture of 2-acetyl furan 23 (0.048 mol), diethyl oxalate24 (0.048 mol), thinly sliced sodium (0.048 mol) in ethanol (55 mL) was first stirred at 0 oC and then stirred overnight at room temperature. After complication, the mixture was acidified (pH 3.0, with 20% H2SO4), filtered and extracted withdichloromethane. The organic phase was dried and concentrated under vacuum to obtain an yellow liquid 25. The ethyl2-(furan-2-yl)-2-oxoacetate 25 (0.025 mol) was dissolved in methanol (10 mL), then added dropwise to a cooled solution (0 oC)of substituted-hydrazine(0.025 mol) in methanol (30 mL). The reaction was stirred for an hour at room temperature, refluxed for2h, and the solvent was evaporated under reduced pressure. The residual liguid was purified by column chromatography (a 5%gradient of ethyl acetate in hexanes over a column of silica gel) to afford immediate 26. To obtain the acid 27, a solution of 26(0.007 mol) was saponified through adding 7 mL 6 mol/L NaOH and stirring at 80 oC for 2 h. The mixture was acidified (pH1-2)with concentrated hydrochloric acid and filtered to afford 42. The Oxalyl chloride0.012 molwas added dropwise to a solutionof acid 42 (0.004 mol) and dimethylformamide (Catalytic amount) in dichloromethane (10 mL)the reaction was stirred for anhour at room temperature for 3 h. NH3·H2O was added dropwise and stirred at room temperature for 0.5h in tetrahydrofuran(15mL) to afford compound 28. A cooled solution (0 oC) solution of thionyl chloride (0.032mmol) in dimethylformamide (25 mL)was added over 30 minutes to a stirred solution of 28, The reaction was stirred for 45minutes at -5 oC and then stirred 2h at roomtemperature. The reaction mixture was poured into ice water (90 mL) and stirred for 0.5 h. A large amount of gray solid wasprecipitated and filtered. The solid was dissolved in ethanol and recrystallized from water to afford 29. Pyrazole 29 (0.043 mol)was dissolved in acetone (120 mL) and KMnO4 (0. 071 mmol) was added. This mixture was heated at 60 oC for 3 h and cooledto room temperature. Then isopropyl alcohol was added and stirred at room temperature overnight. The reaction mixture wasfiltered and concentrated. The residue was dissolved in 1 mol/L NaOH, washed and acidified with 2 mol/L HCl solution toobtain 30. The amide derivatives 31 were prepared through the acyl chlorides derived from 30. A solution of 30 (0.004 mol) inthionyl chloride (10 mL) was refluxed for 5 h and then concentrated under vacuum. The crude acylchloride was added dropwiseto a cooled solution (0 oC) of 2-aminoethanol (0.004 mol) and TEA (0.008 mol) in dichloromethane (10 mL). The mixture wasstirred at room temperature for 0.5 h to afford 31, The compound 6 was added dropwise to a cooled solution (0 oC) of compound31 (0.004 mol) and TEA (0.008 mol) in dichloromethane (10 mL). The mixture was stirred overnight at room temperature, andthen purified on a column of silica using a gradient of ethyl acetate in hexanes to afford the pure products.
References:
Li, Wei;Li, Jiuhui;Shen, Hongfeng;Cheng, Jiagao;Li, Zhong;Xu, Xiaoyong [Chinese Chemical Letters,2018,vol. 29,# 6,p. 911 - 914] Location in patent:supporting information
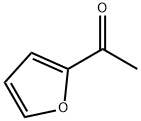
1192-62-7
491 suppliers
$5.00/10g

100537-55-1
19 suppliers
$203.00/500mg
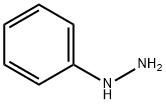
100-63-0
357 suppliers
$10.00/1g

100537-55-1
19 suppliers
$203.00/500mg

104295-62-7
12 suppliers
$60.00/50mg

100537-55-1
19 suppliers
$203.00/500mg
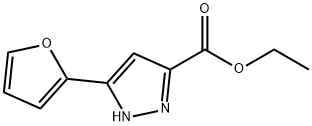
34020-22-9
24 suppliers
$15.00/5mg

100537-55-1
19 suppliers
$203.00/500mg