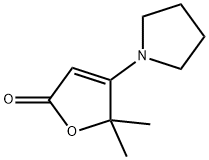
5,5-dimethyl-4-pyrrolidin-1-ylfuran-2-one synthesis
- Product Name:5,5-dimethyl-4-pyrrolidin-1-ylfuran-2-one
- CAS Number:944591-46-2
- Molecular formula:C10H15NO2
- Molecular Weight:181.23
Yield:944591-46-2 92%
Reaction Conditions:
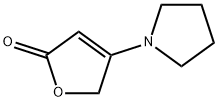
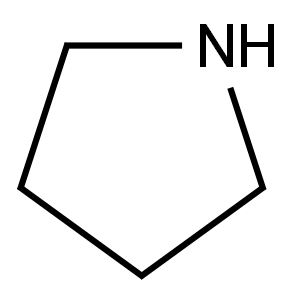
Stage #1: 4-(pyrrolidin-1-yl)-5H-furan-2-onewith lithium hexamethyldisilazane in tetrahydrofuran at -78; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran at -78 - 20; for 1 h;
Stage #3: with water;acetic acid in tetrahydrofuran;
Steps:
Preparation of 5,5-dimethyl-4-pyrrolidin-1-ylfuran-2(5H)-one
Preparation of 5,5-dimethyl-4-pyrrolidin-1-ylfuran-2(5H)-one To a -78° C. stirred suspension of 4-pyrrolidin-1-ylfuran-2(5H)-one (1.0 g, 6.5 mmol) in 20 mL of anhydrous THF, was slowly added 1M LiHMDS/THF solution (14.4 mL, 14.4 mmol). The mixture was stirred at -78° C. under nitrogen for 30 minutes, and methyl iodide (3 mL, 24 mmol) was added. The mixture was stirred at -78° C. for 1 hour, then allowed to warm up to room temp. The reaction was quenched with AcOH (3 mL) and water (10 mL), concentrated to about 15 mL, and extracted with CHCl3 (3*50 mL). The organic layers were combined, washed with 0.5M HCl (2*50 mL), brine (50 mL), dried over Na2SO4, and evaporated to give 5,5-dimethyl-4-pyrrolidin-1-ylfuran-2(5H)-one as a brown solid. Yield: 1.08 g, 92%. 1H NMR (500 MHz, CDCl3) δ ppm 1.54 (s, 6 H) 1.89-1.99 (m, 4 H) 3.32 (br. s., 4 H) 4.33 (s, 1 H)
References:
US2007/173500,2007,A1 Location in patent:Page/Page column 152