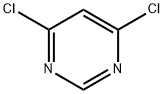
5-(6-Chloropyrimidin-4-yloxy)-1H-indole synthesis
- Product Name:5-(6-Chloropyrimidin-4-yloxy)-1H-indole
- CAS Number:630126-16-8
- Molecular formula:C12H8ClN3O
- Molecular Weight:245.66
Yield:630126-16-8 97.4%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in acetonitrile at 20; for 1 h;
Steps:
3.2 2. Synthesis of 5- (6-chloropyrimidin-4-yloxy) -1H-indole (Compound 16b)
To a solution of 5-hydroxyindole (536.3 mg, 3.6 mmol), 4,6-dichloropyrimidine (399.5 mg, 3.0 mmol) and1,8-diazabicyclo [5.4.0] undec-7-ene (897.3 μL, 6.0 mmol) was stirred at room temperature for 1 hour. The mixture was diluted with ethyl acetate (EtOAc), washed with water and brine, dried over anhydrous MgSO4 and concentrated in vacuo. The thus-obtained crude product was purified by flash chromatography (MeOH: DCM = 1: 40) to obtain Compound 16b (683.8 mg, 2.783 mmol, 92.8%) as a white solid.
References:
KR2016/147170,2016,A Location in patent:Paragraph 0228; 0230; 0233; 0234