
5,6-Dimethoxynicotinaldehyde synthesis
- Product Name:5,6-Dimethoxynicotinaldehyde
- CAS Number:52605-99-9
- Molecular formula:C8H9NO3
- Molecular Weight:167.16

1428797-03-8
1 suppliers
inquiry

52605-99-9
54 suppliers
$161.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 2,3-dimethoxy-5-vinylpyridinewith osmium(VIII) oxide;4-methylmorpholine N-oxide in tetrahydrofuran;water at 25; for 0.5 h;
Stage #2: with sodium periodate in tetrahydrofuran;water at 25; for 12 h;
Steps:
5.2 Step 2: 5, 6-Dimethoxynicotinaldehyde
NMO (3.83g, 32.7mmol) and 0s04 (0.040M in 0, 42mL, l.7mmol) were added to a mixture of 2,3-dimethoxy-5-vinylpyridine (2.70g, l6.3mmol) in THF (60mL) and H2O (l5mL). The reaction mixture was stirred at RT for 30 min. NaI04 (6.99g, 32.7mmol) was added, and the reaction mixture was stirred at RT for 12 hours. The reaction mixture was diluted with H2O (50mL) and EtOAc (50mL). The reaction mixture was extracted with EtOAc (3x50 mL). The organic layers were combined, washed with brine (50mL), dried over MgS04, filtered, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluting EtOAc in PE) to afford 5,6-dimethoxynicotinaldehyde. LCMS (CxHioNOri (ES, m/z): 168 [M+H]+. XH NMR (400MHz, CDCf-ri) 5=9.94 (s, 1H), 8.21 (d, =l.8Hz, 1H), 7.48 (d, J=l.8Hz, 1H), 4.12 (s, 3H), 3.94 (s, 3H).
References:
WO2019/195063,2019,A1 Location in patent:Page/Page column 45; 54-55
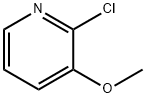
52605-96-6
180 suppliers
$6.00/250mg

52605-99-9
54 suppliers
$161.00/100mg
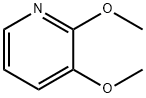
52605-97-7
181 suppliers
$6.00/250mg

52605-99-9
54 suppliers
$161.00/100mg

6636-78-8
436 suppliers
$5.00/1g

52605-99-9
54 suppliers
$161.00/100mg
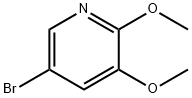
52605-98-8
72 suppliers
$31.00/100mg

52605-99-9
54 suppliers
$161.00/100mg