
5-aMino-1-cyclohexyl-1h-pyrazole-4-carboxaMide synthesis
- Product Name:5-aMino-1-cyclohexyl-1h-pyrazole-4-carboxaMide
- CAS Number:21254-07-9
- Molecular formula:C10H16N4O
- Molecular Weight:208.26
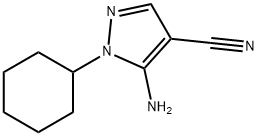
21254-04-6
8 suppliers
inquiry

21254-07-9
4 suppliers
inquiry
Yield:21254-07-9 72%
Reaction Conditions:
Stage #1: 5-amino-1-cyclohexyl-1H-pyrazol-4-carbonitrilewith sulfuric acid at 0 - 25; for 3 h;
Stage #2: with ammonia;water pH=8 - 9;
Steps:
1.2
Step 2: 5-Amino-l -cyclohexyl-lH-pyrazole-4-carboxylic acid amide; [00266] Concentrated sulfuric acid (98 %, 100 mL) was cooled to 0 - 5 0C, and 5-amino-l- cyclohexyl-lH-pyrazole-4-carbonitrile (14.0 g, 73.6 mmol) was added portionwise with vigorous stirring whilst maintaining the temperature below 10 0C . The mixture was stirred at 0 - 5 0C for 2 h, then allowed to warm to 25 0C and stirred at this temperature for 1 h. The mixture was poured onto crushed ice and basified to pη 8-9 by cautious addition of ammonium hydroxide solution. The EPO
References:
WO2008/55959,2008,A1 Location in patent:Page/Page column 62-63

24214-73-1
157 suppliers
$9.00/1g

21254-07-9
4 suppliers
inquiry