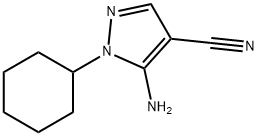
5-AMINO-1-CYCLOHEXYL-1H-PYRAZOLE-4-CARBONITRILE synthesis
- Product Name:5-AMINO-1-CYCLOHEXYL-1H-PYRAZOLE-4-CARBONITRILE
- CAS Number:21254-04-6
- Molecular formula:C10H14N4
- Molecular Weight:190.24
Yield:21254-04-6 87%
Reaction Conditions:
with triethylamine in ethanol at 85; for 18 h;
Steps:
1.1
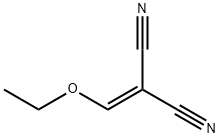
Step 1: 5-Amino-l-cyclohexyl-lH-pyrazole-4-carbonitrile (Intermediate IA; R2 = cyclohexyl, R3 = η); [00265] A solution of (2-ethoxymethylidene)malononitrile (10.35 g, 84.7 mmol), cyclohexylhydrazine hydrochloride (15.2 g, 100.9 mmol) and triethylamine (41 mL, 296.6 mmol) in EtOH (350 mL) was heated at 85 0C for 18 h. The ethanol was evaporated and the residue partitioned between diethyl ether (150 mL) and water (50 mL). The aqueous phase was extracted with diethyl ether (2 x 50 mL). Organic phases were combined, washed with brine (100 mL), dried (MgSO/i) and evaporated. The resulting solid was triturated with diethyl ether and petroleum ether 40-60 0C (1 :20) and the solid was collected by filtration. 5-Amino-l -cyclohexyl- lH-pyrazole-4-carbonitrile (14.0 g, 87 %) was isolated as a yellow solid that was used without any further purification. 1H NMR (400MHz, CDCl3): δ 7.53 (IH, s), 4.56 (2H, s), 3.87 - 3.69 (IH, m), 1.92 - 1.66 (6H, m), 1.63-1.60 (IH, m), 1.46-1.29 (3H, m).
References:
WO2008/55959,2008,A1 Location in patent:Page/Page column 62

108-85-0
338 suppliers
$5.00/10g
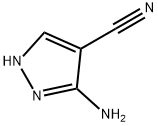
16617-46-2
482 suppliers
$6.00/5g
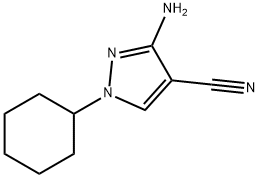
959430-07-0
5 suppliers
inquiry

21254-04-6
8 suppliers
inquiry