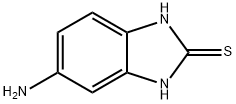
5-Amino-2-benzimidazolethiol synthesis
- Product Name:5-Amino-2-benzimidazolethiol
- CAS Number:2818-66-8
- Molecular formula:C7H7N3S
- Molecular Weight:165.22
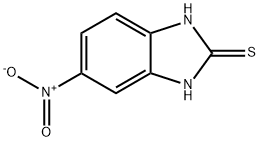
6325-91-3
194 suppliers
$18.00/1g

2818-66-8
226 suppliers
$50.00/10 g
Yield: 80%
Reaction Conditions:
with hydrogenchloride;iron in ethanol;water
Steps:
39.A Synthesis of 2-mercapto-5-(tert-butoxycarbonyl)aminobenzimidazole
A. A mixture of 2-mercapto-5-nitrobenzimidazole (10.0 g, 5 1.23 mmol) and iron fillings (8.0 g, 143.24 mmol) in ethanol (80 mL) and water (10 mL) was refluxed. Then, concentrated HCl (1.2 mL) was added dropwise in ca. 12 min. The resulting dark brown mixture was refluxed for a further 1.5 h then cooled in ice and neutralized with a saturated sodium bicarbonate solution to pH 7.0. The mixture was diluted with EtOH (50 mL), slurried with celite (0.82 g) and filtered over a bed of celite. The cake was washed with EtOH (3*100 mL). The combined filtrate was concentrated in vacuo to afford 9.2 g of a light brown solid. Crystallization from hot water gave the 2-mercapto-5-aminobenzimidazole (6.74 g, 80%) as a light brown solid. 1 H-NMR (DMSO) δ: 4.98 (br. s, 2H), 6.40-6.43 (m, 2H, Ar-H), 6.81-6.85 (d, J=9.0 Hz, 1H, Ar-H), 12.06 (br. s, 1H). 13 C-NMR (DMSO) δ: 165.9 (CS), 144.9, 133.4, 123.6, 109.8, 94.4 IR (KBr, cm-1): 3362, 3295, 3173, 1637, 1622, 1507.
References:
Karimian;Khashayar;Tam;Tim F.;Desilets;Denis;Lee;Sue;Cappelletto;Tullio;Li;Wanren US6093738, 2000, A