
5-AMino-2-cyano-4-Methylpyridine synthesis
- Product Name:5-AMino-2-cyano-4-Methylpyridine
- CAS Number:897733-08-3
- Molecular formula:C7H7N3
- Molecular Weight:133.15
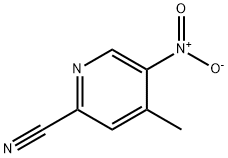
267875-30-9
81 suppliers
inquiry

897733-08-3
22 suppliers
inquiry
Yield:897733-08-3 67%
Reaction Conditions:
with ammonium chloride;zinc in water;ethyl acetate at 0; for 3.5 h;
Steps:
9.37.B
4-methyl-5-nitropicolinonitrile (7.0 g, 43 mmol) was suspended in aq. NH4Cl (200 mL) and cooled to 0° C. Zinc was added portionwise for 30 min and stirred for 1 hr. The reaction was added with ethyl acetate (200 mL) and stirred for 2 hrs. The reaction was filtered and the organic layer was taken up, dried over MgSO4, and concentrated over vacuum. The solid was triturated with 50% ethyl acetate in hexane to give 5-amino-4-methylpicolinonitrile in 67% (4.56 g). 1HNMR (CDCl3, 400 MHz) δ 7.98 (s, 1H), 7.21 (s, 1H), 5.425.48 (b, 2H), 2.54 (s, 3H). Exact mass calculated for C7H7N3 133.15, found 134.21 (MH+).
References:
US2006/155128,2006,A1 Location in patent:Page/Page column 66
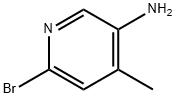
156118-16-0
151 suppliers
$11.00/250mg
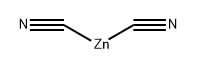
557-21-1
86 suppliers
$12.00/5g

897733-08-3
22 suppliers
inquiry
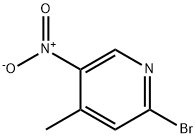
23056-47-5
255 suppliers
$10.00/1g

897733-08-3
22 suppliers
inquiry