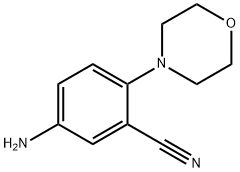
5-amino-2-morpholin-4-ylbenzonitrile synthesis
- Product Name:5-amino-2-morpholin-4-ylbenzonitrile
- CAS Number:78252-12-7
- Molecular formula:C11H13N3O
- Molecular Weight:203.24
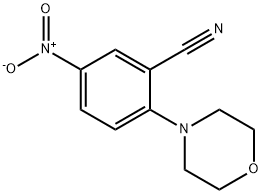
78252-11-6
22 suppliers
inquiry

78252-12-7
17 suppliers
inquiry
Yield: 51.6%
Reaction Conditions:
with tin(II) chloride dihdyrate in ethanol for 6 h;Reflux;
Steps:
10.2 Step 2. Synthesis of 2-morpholinyl-5-aminobenzonitrile (10b):
10a (1.0g, 4.29mmol) was dissolved in 10mL of absolute ethanol,Stannous chloride dihydrate (4.84 g, 21.4 mmol) was added and heated to reflux for 6 h.After the reaction was completed, it was cooled to room temperature, and the reaction mixture was basified with sodium hydrogen carbonate solution and filtered.The filtrate was extracted with ethyl acetate, and the combined organic layers were washed with brine.Dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure.The residue was recrystallized from ethyl acetate to give 10b, 51.6% yield
References:
Hangzhou Yirui Pharmaceutical Technology Co., Ltd.;Wang Xiaolu;Hu Yongzhou;Ye Qing;Hu Xiuai CN109651297, 2019, A Location in patent:Paragraph 0155; 0156; 0159; 0160

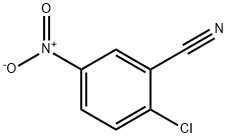
16588-15-1
43 suppliers
$33.39/250mg

78252-12-7
17 suppliers
inquiry
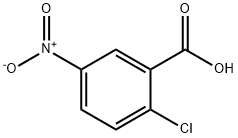
16588-02-6
215 suppliers
$7.00/1g

78252-12-7
17 suppliers
inquiry
2516-96-3
444 suppliers
$6.00/25g

78252-12-7
17 suppliers
inquiry
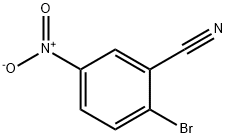
134604-07-2
115 suppliers
$9.00/1g

78252-12-7
17 suppliers
inquiry