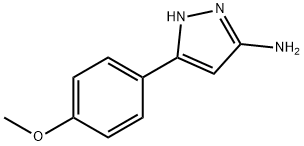
5-AMINO-3-(4-METHOXYPHENYL)PYRAZOLE synthesis
- Product Name:5-AMINO-3-(4-METHOXYPHENYL)PYRAZOLE
- CAS Number:19541-95-8
- Molecular formula:C10H11N3O
- Molecular Weight:189.21
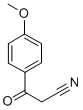
3672-47-7
151 suppliers
$16.00/500mg

19541-95-8
99 suppliers
$12.00/500mg
Yield:19541-95-8 97%
Reaction Conditions:
with hydrazine in ethanol at 80; for 15 h;
Steps:
1
To a solution of 3- (4-methoxyphenyl)-3-oxo-propionitrile (2.0 g, 11.4 mmol) in absolute ethanol (21 mL) was added hydrazine hydrate (3.32 mL, 68.3 mmol). The reaction mixture was stirred at 80°C for 15 hours, then it was concentrated in vacuo and purified on silica gel with 0-10% MeOH in CH2Cl2 as eluent to give 2.02 g (97% yield) of 5-amino-3- (4-methoxyphenyl) pyrazole as a white solid. lH NMR (d6-DMSO) 6 : 11.69 (s, 1H), 7.55 (d, 2H), 6.92 (d, 2H), 5.66 (s, 1H), 4.62 (broad s, 2H), 3.75 (s, 3H); HPLC/MS m/z: 190. 1 [MH] +. To a solution of 5-amino-3- (4-methoxyphenyl) pyrazole (2.5 g, 13.2 mmol) in THF (50 mL) was added dropwise benzoyl isothiocyanate (1.96 mL, 14.5 mmol). The reaction mixture was stirred at room temperature for 45 minutes, then 4 N aqueous solution of NaOH (10 mL) was added, and the reaction mixture was further stirred at 55°C for 1 hour. The reaction mixture was cooled to room temperature, neutralized to pH 8 with 1 N aqueous HCl, and extracted with EtOAc (3x). The combined organic layers were washed with brine, dried over sodium sulfate, filtered, evaporated, and dried in vacuo to provide 4.28 g of an orange-yellow solid. The solid was dissolved in glacial AcOH (300 mL) and a 1.5 M solution of bromine in AcOH (8.8 mL, 13.2 mmol) was added dropwise under vigorous stirring. The resulting heterogeneous mixture was stirred at room temperature for 1 hour then at 80°C for 1 hour. The reaction was cooled to room temperature and Et2O (500 mL) was added. The resulting precipitate was filtered, washed with Et20, and dried in vacuo to afford 2.93 g (68% yield) of 3-(4-methoxyphenyl)-1H-pyrazolo [3, 4-d] thiazol-5-ylamine as a light yellow solid. lH-NMR (d6-DMSO) d : 7.57 (d, 2H), 7.09 (d, 2H), 3.80 (s, 3H) ; HPLC/MS m/z: 247.1 [MH] +. To a vial charged with 3- (4-methoxyphenyl)-lH-pyrazolo [3, 4-d] thiazol-5-ylamine (100 mg, 0. 306 mmol), PS-DMAP (Argonaut resin, 1.2 g, 6 equiv. ), and a stirring bar was added THF (3 mL) and acetyl chloride (110 RL, 1. 53 mmol). The reaction mixture was stirred at 70°C for 1 hour, then cooled to room temperature and treated with PS-trisamine (Argonaut resin, 1. 1 g, 20 equiv. ) for 1 hour. The resin was filtered, washed with THF and the solvent was evaporated. The resulting crude was suspended in MeOH and treated with hydrazine monohydrate (0.06 mL) for 30 minutes. The precipitate was filtered, and the filtrate was directly purified on silica gel with 0-10% MeOH in CH2C12 as eluent to provide 12.5 mg (14% yield) of N-[3-(4-methoxy-phenyl)-1H-pyrazolo [3,4-d] thiazol-5-yl] - acetamide as an off-white solid. lH-NM : R (d6-DMSO) d : 13.4 (broad s, 1H), 12.3 (broad s, 1H), 7.66 (broad s, 2H), 7.09 (broad s, 2H), 3.80 (s, 3H), 2. 18 (s, 3H); HPLC/MS m/z: 289.0 [MH] +.
References:
WO2005/68473,2005,A1 Location in patent:Page/Page column 49-50

35070-82-7
6 suppliers
inquiry

19541-95-8
99 suppliers
$12.00/500mg

57466-68-9
8 suppliers
inquiry

19541-95-8
99 suppliers
$12.00/500mg
90799-51-2
0 suppliers
inquiry

19541-95-8
99 suppliers
$12.00/500mg
121-98-2
279 suppliers
$12.00/1mg

19541-95-8
99 suppliers
$12.00/500mg