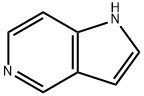
5-Azaindole synthesis
- Product Name:5-Azaindole
- CAS Number:271-34-1
- Molecular formula:C7H6N2
- Molecular Weight:118.14
271-29-4
266 suppliers
$9.00/250mg

98-59-9
585 suppliers
$9.00/5g

271-34-1
310 suppliers
$9.00/250mg
Yield:271-34-1 58%
Reaction Conditions:
Stage #1: 6-azaindolewith potassium hydroxide;iodine in N,N-dimethyl-formamide at 20; pH=10; for 1.75 h;
Stage #2: with sodium hydride in tetrahydrofuran at 20; for 0.25 h;
Stage #3: p-toluenesulfonyl chloride in tetrahydrofuran at 20; for 2 h;
Steps:
To a mixture of 6-azaindole (CAS 271-341) (1.72 g) and KOH (2.4 g) in dry DMF(50 ml) under nitrogen was added iodine (3.73 g) portionwise over 15 min. The mixture was stirred at r.t. for a further 90 min and poured into water (600 ml) containing sodium metabisulfite solution (100 ml). Adjusting the solution to pHIO caused the product to precipitate out. The solid was extracted with EtOAc (500 ml). The organic layer was separated, dried over MgSO4, filtered and the solvent removed in vacuo to yield a yellow solid (2.98 g). This solid was dissolved in dry THF (50 ml) and to the mixture was added portionwise NaH (60% dispersion in mineral oil) (630 mg). The mixture was stirred for a further 15 min. Pαrα-toluenesulfonyl chloride (2.42 g) was added in one portion and the mixture stirred at r.t. for 2 hours. The mixture was poured into water (600 ml) and extracted with EtOAc (400 ml). The organic layer was separated, dried over MgSO4, filtered and the solvent removed in vacuo. Purification by column chromatography on silica eluting with 0-10% MeOH/DCM yielded the title compound as a solid (3.37 g, 58%). LCMS 399 [M+H]+, RT 2.99 min. 1H NMR 300 MHz (CDCl3) 8.70 (1H, s), 8.55 (1H, d), 7.90-7.75 (3H, m), 7.70 (1H, s), 7.30 (2H, d), 2.40 (3H, s).
References:
WO2006/38001,2006,A1 Location in patent:Page/Page column 51