
5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL synthesis
- Product Name:5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL
- CAS Number:23288-90-6
- Molecular formula:C9H8N2OS
- Molecular Weight:192.24

75-15-0
265 suppliers
$40.00/100g
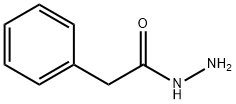
937-39-3
131 suppliers
$10.00/1g
![5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL](/CAS/GIF/23288-90-6.gif)
23288-90-6
20 suppliers
$45.00/25mg
Yield:23288-90-6 93%
Reaction Conditions:
with sodium hydroxide in ethanol; for 4 h;Reflux;
Steps:
General procedure: Method A: With reference to Manjunatha et a/., the 1 ,3,4-oxadiazole derivatives were synthesized as described below:[6] The appropriate hydrazide (10.0 mmol) was dissolved in ethanol 96% (30 mL) with addition of sodium hydroxide (0.400 g, 10.0 mmol). After the addition of carbon disulfide 0.776 g, 10.2 mmol) the reaction batch was refluxed for four hours. The mixture was cooled to room temperature. Addition of 1 M hydrochloric acid (10.0 mL) led to precipitation. The precipitates were filtered, washed with water and recrystallized from ethanol. 5-Benzyl-1 ,3,4-oxadiazole- -thiol:[10] The compound was synthesized as described in method A; light yellow powder (Yield: 93%). Mp = 125 - 127 °C. (129 - 130 °Ct10]) 1H-NMR (DMSO-de, 400 MHz): 4.14 (s, 2H, CH2), 7.28-7.41 (m, 5H, ArH), 14.12 (s, br, 1 H, SH). 13C-NMR (DMSO-d6, 100 MHz): 31.0 (sec. C); 127.4, 128.8 (2C), 129.0 (2 C) (tert. C); 133.5, 163.0, 177.8 (quat. C). MS (El): m/z (%) = 192 [Mf (69).
References:
WO2014/79545,2014,A1 Location in patent:Page/Page column 36; 37; 38

101-41-7
524 suppliers
$16.80/100ML
![5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL](/CAS/GIF/23288-90-6.gif)
23288-90-6
20 suppliers
$45.00/25mg
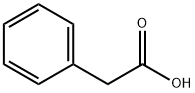
103-82-2
0 suppliers
$22.60/100g
![5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL](/CAS/GIF/23288-90-6.gif)
23288-90-6
20 suppliers
$45.00/25mg
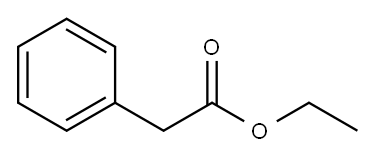
101-97-3
476 suppliers
$14.00/25mL
![5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL](/CAS/GIF/23288-90-6.gif)
23288-90-6
20 suppliers
$45.00/25mg

937-39-3
131 suppliers
$10.00/1g
![5-BENZYL-[1,3,4]OXADIAZOLE-2-THIOL](/CAS/GIF/23288-90-6.gif)
23288-90-6
20 suppliers
$45.00/25mg