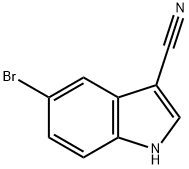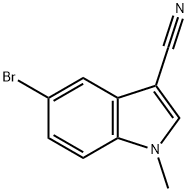
5-bromo-1-methyl-1H-indole-3-carbonitrile synthesis
- Product Name:5-bromo-1-methyl-1H-indole-3-carbonitrile
- CAS Number:1219741-43-1
- Molecular formula:C10H7BrN2
- Molecular Weight:235.08
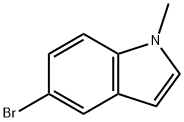
10075-52-2
114 suppliers
$45.00/100mg
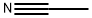
75-05-8
966 suppliers
$10.00/10g

1219741-43-1
5 suppliers
inquiry
Yield:1219741-43-1 92%
Reaction Conditions:
Stage #1: 5-bromo-1-methyl-H-indole;acetonitrilewith isocyanate de chlorosulfonyle at 0; for 2 h;
Stage #2: in N,N-dimethyl-formamide at 0; for 1 h;
Steps:
General Procedure for the Synthesis of 1-methyl-1H-indole-3-carbonitriles (5a-e)
General procedure: The appropriate indole 4a-e (1.8 mmol) solubilized in anhydrous acetonitrile (2.0 mL), was reacted with chlorosulfonyl isocyanate (CSI) (0.16 mL, 1.8 mmol, added dropwise at 0 °C. The resulting reaction mixture was stirred at 0 °C for 2 h after which anhydrous dimethylformamide (DMF) (1.0 mL,106.3 mmol) was added dropwise. The mixture was stirred at 0 °C for 1 h and then it was poured into ice-water. The obtained precipitate was filtered off, dried (Na2SO4), and purified by column chromatography using dichloromethane (DCM) as the eluent.
References:
Cascioferro, Stella;Attanzio, Alessandro;Di Sarno, Veronica;Musella, Simona;Tesoriere, Luisa;Cirrincione, Girolamo;Diana, Patrizia;Parrino, Barbara [Marine Drugs,2019,vol. 17,# 1,art. no. 35]

10075-52-2
114 suppliers
$45.00/100mg
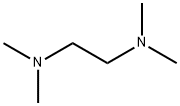
110-18-9
527 suppliers
$10.00/5mL

1219741-43-1
5 suppliers
inquiry

10075-52-2
114 suppliers
$45.00/100mg
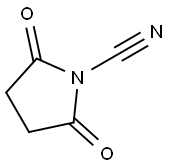
135205-66-2
8 suppliers
inquiry

1219741-43-1
5 suppliers
inquiry