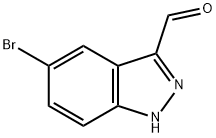
5-BROMO-1H-INDAZOLE-3-CARBALDEHYDE synthesis
- Product Name:5-BROMO-1H-INDAZOLE-3-CARBALDEHYDE
- CAS Number:201227-38-5
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04
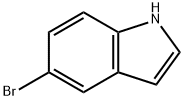
10075-50-0
672 suppliers
$5.00/10g

201227-38-5
151 suppliers
$45.00/50mg
Yield:201227-38-5 76%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water;acetone at -10 - 20; for 3 h;
Steps:
1
A solution of NaNO2 (110.4 g, 1.6 mol, 8 eq) in water (200 mL) was added dropwise to a solution of 5-bromoindole (XI) (39.2 g, 0.2 mol, 1 eq) in acetone (1000 mL) stirred at -10→0° C., while adding NaNO2 the solution temperature was maintained below 20° C. An aqueous 2N HCl solution (480 mL) was added slowly to the solution with vigorously stirring while keeping the internal temperature between 0 and 20° C. The solution was further stirred at 20° C. for 3 h after the addition. The solution was concentrated under reduced pressure to remove acetone while keeping the temperature below 35° C. The solid was collected by filtration and transferred to a flask. Cold (-10° C.) DCM (200 mL) was added and stirred for 30 min at -5° C., the solids were filtered and dried under vacuum at 40° C. to get 5-bromo-1H-indazole-3-carbaldehyde (XII) (34.0 g, 151 mmol, 76% yield) as a brown solid. ESIMS found for C8H5BrN2O m/z 225 (M+H).
References:
Samumed, LLC;KC, Sunil Kumar;Wallace, David Mark;Cao, Jianguo;Chiruta, Chandramouli;Hood, John US2016/75701, 2016, A1 Location in patent:Paragraph 0798; 0799