
5-bromo-2-(2-methylpropoxy)Pyridine synthesis
- Product Name:5-bromo-2-(2-methylpropoxy)Pyridine
- CAS Number:1251385-87-1
- Molecular formula:C9H12BrNO
- Molecular Weight:230.1
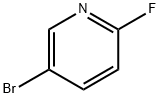
766-11-0
459 suppliers
$6.00/5g
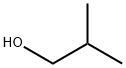
78-83-1
802 suppliers
$14.00/5mL

1251385-87-1
13 suppliers
$388.00/1g
Yield:1251385-87-1 86%
Reaction Conditions:
with caesium carbonate in dimethyl sulfoxide at 120; for 18 h;
Steps:
23.A Part A: 5 -Bromo-2-isobutoxypyridine
A mixture of cesium carbonate (1.977 g, 6.07 mmol), 5-bromo-2-fluoropyridine (1.07 g, 6.07 mmol) and 2-methylpropan-1-ol (0.899 g, 12.13 mmol)in DMSO (5 mL) was stirred at 120 °C for 18 hours. The reaction was diluted with water and extract with diethyl ether three times. The diethyl ether layers were combined, dried (Na2504), filtered and concentrated. The residue was purified via silica gel flash column chromatography eluting with ethyl acetate in hexane from 0 to10% to give the desired product (1.20 g, 86% yield). ‘H NMR (400MHz,CHLOROFORM-d) ? 8.21 -8.15 (m, 1H), 7.64 (dd,J8.8, 2.5 Hz, 1H), 6.66 (dd,J=8.8, 0.5 Hz, 1H), 4.03 (d, J=6.5 Hz, 2H), 2.08 (dt, J13.4, 6.7 Hz, 1H), 1.01 (d,J=6.8 Hz, 6H); LCMS (ESI) m/e 173.9 [(M-isobutyl +H), calcd C5H5Br1N1O1,173.9j; LC/MS retention time (method A): tR = 2.01 mm.
References:
WO2017/184658,2017,A1 Location in patent:Page/Page column 42