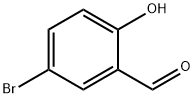
5-BROMO-2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE synthesis
- Product Name:5-BROMO-2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE
- CAS Number:428482-55-7
- Molecular formula:C14H10BrClO2
- Molecular Weight:325.58
Yield:428482-55-7 100%
Reaction Conditions:
Stage #1: 5-bromo-2-hydroxybenzaldehydewith potassium carbonate in DMF (N,N-dimethyl-formamide) at 20; for 0.5 h;
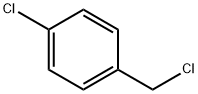
Stage #2: p-chlorobenzyl chloride in DMF (N,N-dimethyl-formamide) at 65;
Steps:
1 Example 1; 5-Bromo-2-(4-chlorophenylmethoxy)benzaldehyde
To a stirred solution of 5-bromo-2-hydroxybenzaldehyde (100 g, 0.5 mol) in DMF (600 mL) was added K2CO3 (206 g, 1.49 mol) at rt. After 30 min, 4-chlorobenzylchloride (78 g, 0.48 mol) was added. The reaction mixture was kept at 65° C. overnight, then cooled to rt, and poured into an ice-cooled mixture of EtOAc/water (1:1, 2 L). The solid was collected by filtration, washed with water, and dried under vacuum for 20 h to give the desired product (160 g, 100%). 1H NMR (400 MHz, CDCl3): δ 5.18 (s, 2H), 6.92 (d, 1H), 7.37 (m, 4H), 7.61 (d, 1H), 7.98 (s, 1H), 10.4 (s, 1).
References:
US2005/101644,2005,A1 Location in patent:Page/Page column 32