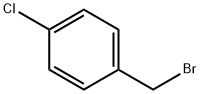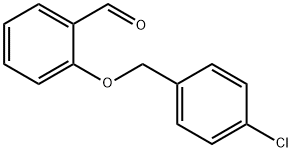
2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE synthesis
- Product Name:2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE
- CAS Number:52803-59-5
- Molecular formula:C14H11ClO2
- Molecular Weight:246.69

90-02-8
445 suppliers
$9.00/1g
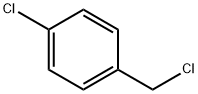
104-83-6
371 suppliers
$15.00/50g
![2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE](/CAS/GIF/52803-59-5.gif)
52803-59-5
22 suppliers
$45.00/50mg
Yield:52803-59-5 87%
Reaction Conditions:
with potassium carbonate;potassium iodide in ethanol at 85;
Steps:
Synthesisof intermediate 3-8, 30, 32, 34 and 38:
General procedure: To a solution of the different substituted hydroxybenzaldehyde (2a-2e) (4 mmol) in ethanol (30 ml), K2CO3(1 eq), KI (1 eq) and substituted benzyl chloride (1a-1e) (4 mmol) were added at room temperature. The reactionmixture was refluxed at 85°C for 4-8 hours. After completion, the solvent wasremoved in vacuo. Water (100 ml) was added and the mixture was extracted withethyl acetate, and evaporated. The crude oil was puried by columnchromatography over silica gel (petroleum ether : ethyl acetate = 20:1), as awhite solid, yield 63%-87%.
References:
Zhao, Shuangmei;Zhen, Yongqi;Fu, Leilei;Gao, Feng;Zhou, Xianli;Huang, Shuai;Zhang, Lan [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 19,art. no. 126623] Location in patent:supporting information

90-02-8
445 suppliers
$9.00/1g
![2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE](/CAS/GIF/52803-59-5.gif)
52803-59-5
22 suppliers
$45.00/50mg
89-95-2
202 suppliers
$22.00/10g

104-83-6
371 suppliers
$15.00/50g
![2-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE](/CAS/GIF/52803-59-5.gif)
52803-59-5
22 suppliers
$45.00/50mg