5-bromo-2-(4-methoxyphenyl)pyridine synthesis
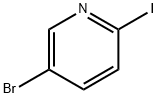
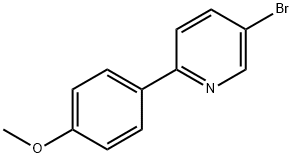
- Product Name:5-bromo-2-(4-methoxyphenyl)pyridine
- CAS Number:88345-93-1
- Molecular formula:C12H10BrNO
- Molecular Weight:264.12
Yield:88345-93-1 97%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in water;toluene at 80; for 40.5 h;Inert atmosphere;
Steps:
90.1
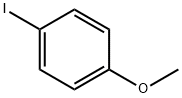
Step 1: Preparation of 5-bromo-2-(4-methoxyphenyl)pyridine A mixture of 5-bromo-2-iodopyridine (600 mg, 2.11 mmol), (4-methoxyphenyl)boronic acid (353 mg, 2.32 mmol), tetrakis(triphenylphosphine)palladium(0) (122 mg, 0.106 mmol), and 2M Na2CO3 aq. (2.11 mL, 4.22 mmol) in toluene (10 mL) was heated at 80° C. for 40.5 hours under nitrogen. After cooling to room temperature, water was added and extracted with EtOAc. The organic layer was washed with brine, dried over Na2SO4 and filtered through a Celite pad. The filtrate was concentrated and the residue was purified by column chromatography (CH2Cl2 only) to afford the desired product (538 mg, 97%) as a yellow solid. 1HNMR (CDCl3) 400 MHz δ: 8.69 (d, J=2.3 Hz, 1H), 7.92 (dt, J=8.6 Hz and 2.3 Hz, 2H), 7.82 (dd, J=8.6 Hz and 2.3 Hz, 1H), 7.56 (d, J=8.6 Hz, 1H), 6.99 (dt, J=8.6 Hz and 2.3 Hz, 2H), 3.87 (s, 3H). LCMS: 264 [M+H].
References:
US2012/108574,2012,A1 Location in patent:Page/Page column 132
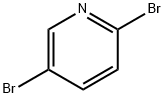
624-28-2
723 suppliers
$5.00/5g
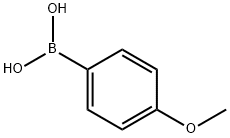
5720-07-0
466 suppliers
$6.00/5g
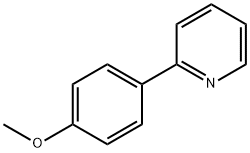
5957-90-4
84 suppliers
$39.00/1g

88345-93-1
20 suppliers
inquiry