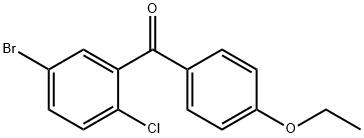
(5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone synthesis
- Product Name:(5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone
- CAS Number:461432-22-4
- Molecular formula:C15H12BrClO2
- Molecular Weight:339.61
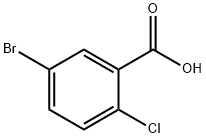
21739-92-4
648 suppliers
$8.00/10g
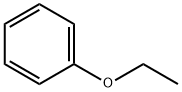
103-73-1
334 suppliers
$8.00/25g

461432-22-4
334 suppliers
$5.00/250mg
Yield:461432-22-4 178 g
Reaction Conditions:
Stage #1:5-bromo-2-chlorobenzoic acid with thionyl chloride;N,N-dimethyl-formamide in dichloromethane at 25 - 40; for 4 h;
Stage #2: with aluminum (III) chloride in dichloromethane at 5 - 30; for 0.166667 h;
Stage #3:Phenetole in dichloromethane at 25 - 30; for 10 h;
Steps:
1 Preparation of (5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone (Formula-3)
Thionyl chloride (194.78 ml) was slowly added to a mixture of 5-bromo-2-chlorobenzoic acid compound of formula-2 (200 gms), dichloromethane (1000 ml) and dimethylformamide (1 ml) at 25-30° C.
Heated the reaction mixture to 35-40° C. and stirred for 4 hrs at the same temperature.
Distilled off the solvent completely from the reaction mixture under reduced pressure.
Dichloromethane (1600 ml) was added to the obtained compound at 25-30° C. and stirred for 20 mins at the same temperature.
Cooled the reaction mixture to 5-10° C. and stirred for 15 mins at the same temperature.
Aluminium chloride (110.9 gms) was slowly added to the reaction mixture at 5-10° C., the temperature of the reaction mixture was raised to 25-30° C. and stirred for 10 mins at the same temperature.
Phenetole (103.5 gms) was slowly added to the reaction mixture at 25-30° C. and stirred for 10 hrs at the same temperature.
The reaction mixture was poured into chilled hydrochloric acid solution (1000 ml of hydrochloric acid in 1000 gms of ice) at 25-30° C. and stirred for 20 mins at the same temperature.
Separated the both organic and aqueous layers, the organic layer was washed with 5% aqueous sodium bicarbonate solution followed by with 10% aqueous sodium chloride solution.
Distilled off the solvent completely from the organic layer under reduced pressure.
Methanol (400 ml) was added to the obtained compound at 55-60° C. and stirred for 45 mins.
Cooled the reaction mixture to 0-5° C. and stirred for 2 hrs at the same temperature.
Filtered the precipitated solid, washed with methanol.
Methanol (500 ml) was added to the wet solid, heated to 65-70° C. and stirred for 1 hr 30 mins at the same temperature.
Cooled the reaction mixture to 25-30° C., then to 0-5° C. and stirred for 3 hrs at 0-5° C.
Filtered the precipitated solid, washed with methanol and then dried to get title compound.
Yield: 178 gms; Melting range: 68-72° C.; Purity by HPLC: 98.8%.
References:
MSN LABORATORIES PRIVATE LIMITED;Thirumalai Rajan, Srinivasan;Eswaraiah, Sajja US2017/29398, 2017, A1 Location in patent:Paragraph 0075-0076

21900-52-7
79 suppliers
$12.00/250mg

103-73-1
334 suppliers
$8.00/25g

461432-22-4
334 suppliers
$5.00/250mg
915095-85-1
152 suppliers
inquiry

64-17-5
717 suppliers
$10.00/50g

461432-22-4
334 suppliers
$5.00/250mg

915095-85-1
152 suppliers
inquiry
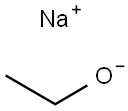
141-52-6
461 suppliers
$10.00/5g

461432-22-4
334 suppliers
$5.00/250mg

462-06-6
414 suppliers
$10.00/5g

21900-52-7
79 suppliers
$12.00/250mg

461432-22-4
334 suppliers
$5.00/250mg