
Dapagliflozin synthesis
- Product Name:Dapagliflozin
- CAS Number:461432-26-8
- Molecular formula:C21H25ClO6
- Molecular Weight:408.88
461432-25-7
296 suppliers
$65.00/100mg

461432-26-8
681 suppliers
$5.00/5mg
Yield:461432-26-8 96.18%
Reaction Conditions:
with lithium hydroxide monohydrate;anhydrous sodium carbonate in methanol at 25 - 50; for 6 h;Time;Reagent/catalyst;
Steps:
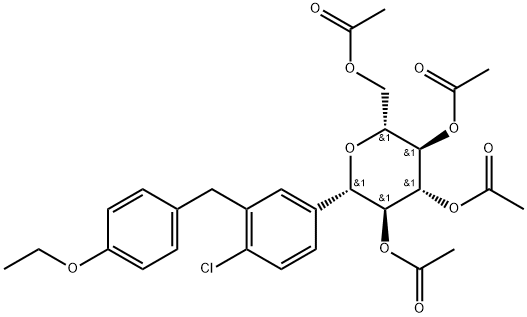
7 Preparation of (1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol Compound of Formula-1 Without Glycerol Solvate Formation Using Sodium Carbonate as a Base for Deacetylation
A mixture of (2R,3R,4R,5S,6S)-2-(acetoxymethyl)-6-(4-chloro-3 -(4-ethoxybenzyl) phenyptetrahydro-2H-pyran-3,4,5-triyl triacetate compound of formula-7 (10 gms), methanol (90 ml) and water (10 ml) was stirred for 30 mins at 25-30° C. Sodium carbonate (16.53 gms) was added to the reaction mixture, heated to 45-50° C. and stirred for 6 hrs at the same temperature.
Cooled the reaction mixture to 25-30° C. and stirred for 15 mins at the same temperature.
Filtered the reaction mixture, washed with methanol and distilled off the solvent completely from the filtrate under reduced pressure.
Ethyl acetate followed by water were added to the obtained compound at 25-30° C. and stirred for 15 mins at the same temperature.
Separated the both organic and aqueous layers, the organic layer was washed with 2% aqueous sodium bicarbonate solution, followed by 10% aqueous sodium chloride solution.
Distilled off the solvent completely from the organic layer under reduced pressure to get (1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol compound of formula-1.
Yield: 6.8 gms; % yield: 96.18%; Purity by HPLC: 99.08%.
References:
US2017/29398,2017,A1 Location in patent:Paragraph 0086-0088

461432-24-6
57 suppliers
inquiry

461432-26-8
681 suppliers
$5.00/5mg
![L-Proline, compd. with (1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol (1:1)](/CAS/20211123/GIF/960404-66-4.gif)
960404-66-4
0 suppliers
inquiry

461432-26-8
681 suppliers
$5.00/5mg
![D-Glucopyranose, 1-C-[4-chloro-3-[(4-ethoxyphenyl)Methyl]phenyl]-2,3,4,6-tetrakis-O-(triMethylsilyl)-](/CAS/20210111/GIF/960494-15-9.gif)
960494-15-9
8 suppliers
inquiry

461432-26-8
681 suppliers
$5.00/5mg

714269-57-5
186 suppliers
inquiry

461432-26-8
681 suppliers
$5.00/5mg