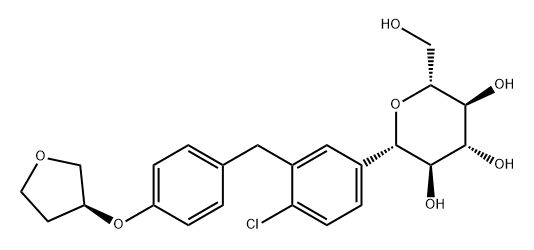
Empagliflozin synthesis
- Product Name:Empagliflozin
- CAS Number:864070-44-0
- Molecular formula:C23H27ClO7
- Molecular Weight:450.91
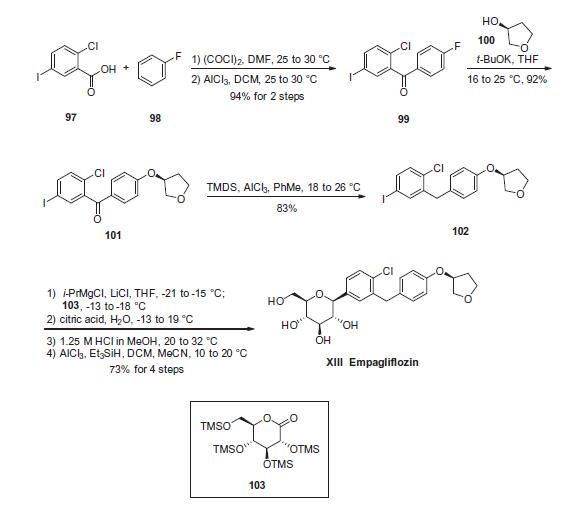

1279691-36-9
164 suppliers
inquiry

864070-44-0
646 suppliers
$5.00/10mg
Yield:864070-44-0 82.5%
Reaction Conditions:
with triethylsilane;Aluminum Chloride in dichloromethane;acetonitrile;
Steps:
3 (2S,3R,4S,5R,6R)-2-(4-chloro-3- (4-(((S) -tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-6- (hydroxymethyl) tetrahydro-2H-pyran-3,4,5-triol
Add acetonitrile: dichloromethane (1: 1,200mL by volume) to a 1L dry reaction flask.Stir down to 0 10 , then add 42g in orderAluminum trichloride and 32.5gTriethylsilane,The reaction was stirred for 30 min.50g(2S, 3R, 4S, 5R, 6R) -2- (4-chloro-3- (4-(((S) -tetrahydrofuran-3-yl) oxy) benzyl) phenyl) -6- (hydroxymethyl ) 2-methoxytetrahydro-2H-pyran-3,4,5-triol in acetonitrile: dichloromethane(Volume ratio 1: 1, 200 mL) The solution was dropped into the above reaction system, and the dropping was completed in 1 to 2 hours;The temperature was controlled at 20 to 30 ° C and the reaction was stirred for 1 to 2 hours. Add 400mL of water,The organic solvent was distilled off under reduced pressure, and the reaction solution was extracted with 200 mL × 2 ethyl acetate.The organic phases were combined, washed once with saturated brine, and concentrated under reduced pressure to give a pale yellow oil.Purity 90.5%.Add 50 mL of ethyl acetate to the concentrate, turn on the stirring, and lower the temperature to -40 ° C to -50 ° C.After precipitation of a large amount of white solid, stirred for 0.5 h, and 250 mL of n-heptane was added dropwise to the reaction.Stir for 0.5h, filter with suction, and place the filter cake in a hot air circulation drying box for 15h.38.7 g of the title compound was obtained, with a molar yield of 82.5% and a purity of 98.3%.
References:
CN110407891,2019,A Location in patent:Paragraph 0043-0048
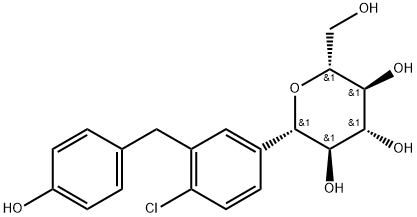
864070-37-1
87 suppliers
inquiry
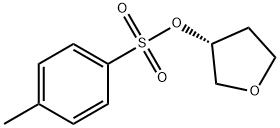
219823-47-9
113 suppliers
inquiry

864070-44-0
646 suppliers
$5.00/10mg
![D-Glucitol, 1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-](/CAS/20211123/GIF/1417573-74-0.gif)
1417573-74-0
1 suppliers
inquiry

864070-44-0
646 suppliers
$5.00/10mg