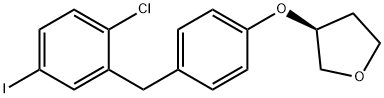
(S)-3-(4-(2-Chloro-5-iodobenzyl)phenoxy)tetrahydrofuran synthesis
- Product Name:(S)-3-(4-(2-Chloro-5-iodobenzyl)phenoxy)tetrahydrofuran
- CAS Number:915095-94-2
- Molecular formula:C17H16ClIO2
- Molecular Weight:414.67
![(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone](/CAS/20150408/GIF/915095-87-3.gif)
915095-87-3
173 suppliers
inquiry

915095-94-2
365 suppliers
inquiry
Yield:915095-94-2 90%
Reaction Conditions:
with aluminum (III) chloride;sodium tetrahydroborate in tetrahydrofuran for 5 h;Reflux;
Steps:
3
Equipped with a thermometer, a reflux condenser, a 500 mL three-necked flask with a drying tube was added VI (50g, 116.5mmol), THF (350mL), stirring at room temperature was added NaBH4(22.0g, 582.5mmol), was added portionwise AlCl3(39g, 291.5mmol), with vigorous bubbling system, the addition was complete the reaction was heated at reflux for 5 h, TLC detection starting material the reaction was complete, the reaction was stopped.A system was cooled to room temperature and slowly poured into 200mL ice water + 75mL 2MHCl stirred vigorously for 0.5h, allowed to stand separated and the aqueous phase was extracted with ethyl acetate (100mL × 3). The combined organic phases were washed with sodium bicarbonate solution until neutral, saturated sodium chloride (200 mL) washed, dried and concentrated to give the crude product as a pale yellow solid, water was added 50mL + 10mL of ethanol as a white solid crystallized to give 26.6g IV of them, in 90% yield.
References:
Shanghai Institute of Technology;Pan, Xianhua;ZHANG, XIN;ZHANG, RONG;Cao, Yang;CHEN, YANYU;LI, HONGYI;PENG, QIUJUN CN105399735, 2016, A Location in patent:Paragraph 0043; 0044; 0045
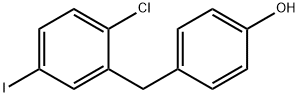
1459754-32-5
41 suppliers
inquiry
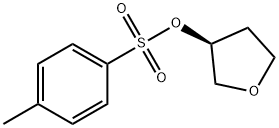
112052-11-6
48 suppliers
inquiry

915095-94-2
365 suppliers
inquiry
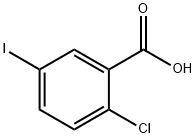
19094-56-5
513 suppliers
$6.00/5g

915095-94-2
365 suppliers
inquiry
915095-86-2
209 suppliers
$5.00/250mg

915095-94-2
365 suppliers
inquiry

462-06-6
411 suppliers
$10.00/5g

915095-94-2
365 suppliers
inquiry