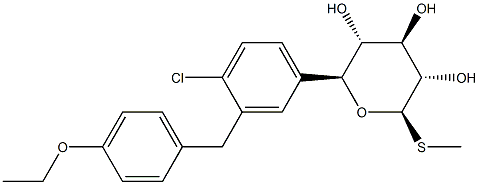
LX-4211 synthesis
- Product Name:LX-4211
- CAS Number:1018899-04-1
- Molecular formula:C21H25ClO5S
- Molecular Weight:424.94
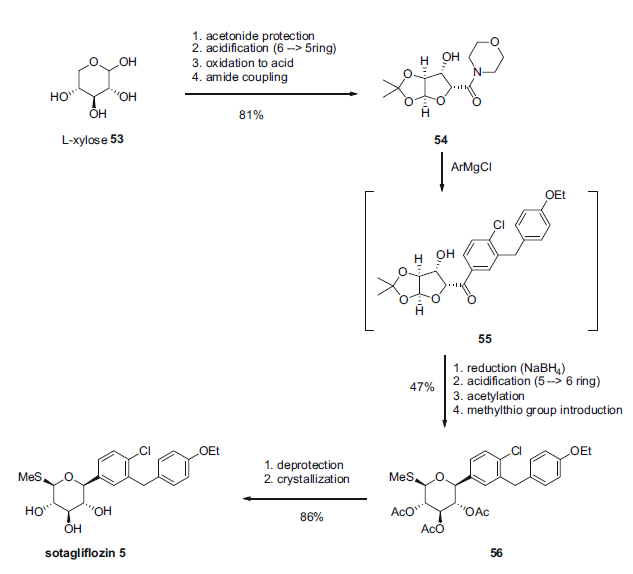
Sotagliflozin synthesis
![β-L-Xylopyranoside, Methyl 5-C-[4-chloro-3-[(4-ethoxyphenyl)Methyl]phenyl]-1-thio-, 2,3,4-triacetate,(5S)-](/CAS/20150408/GIF/1018899-03-0.gif)
1018899-03-0
37 suppliers
inquiry

1018899-04-1
221 suppliers
$29.00/1mg
Yield:1018899-04-1 99.7%
Reaction Conditions:
with methanol;sodium methylate in toluene at 113; under 13501.4 Torr; for 0.00555556 h;Temperature;Pressure;
Steps:
1; 2; 3 Synthesis of sotagliflozin in toluene
Sotagliflozin was synthetized in an intensified reactor at 113°C under a pressure of 8 bar.The reactor was fed with the solution of compound (A) in 10 Volumes of toluene with a flow of 1194.6 g/h and the methanolic solution of MeONa (0.6 equivalent of MeONa and 15 equivalents of MeOH) with a flow of 114.6 g/h, which were preheated at 113°C.The time of residence in the reactor was 20 seconds.The system was maintained at a pressure of 8 bar.Sotagliflozin was synthetized with a yield of 99.7%.Aqueous washing, dehydration, crystallization, filtration, washing, drying were performed according to example 2.An XRPD (X-ray diffraction) analysis confirmed that Form II of sotagliflozin was obtained.
References:
LEXICON PHARMACEUTICALS, INC. WO2021/19507, 2021, A1 Location in patent:Page/Page column 9-12
67-63-0
1551 suppliers
$16.00/25ML
114861-22-2
87 suppliers
$40.00/250mg

1018899-04-1
221 suppliers
$29.00/1mg
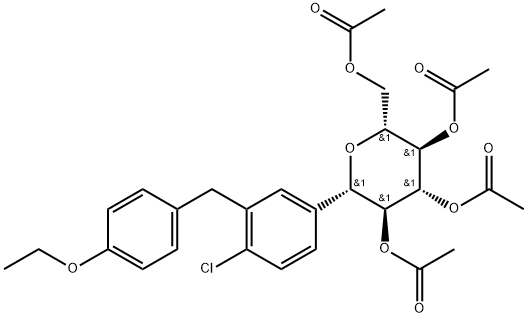
461432-25-7
279 suppliers
$65.00/100mg

1018899-04-1
221 suppliers
$29.00/1mg
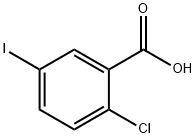
19094-56-5
511 suppliers
$6.00/5g

1018899-04-1
221 suppliers
$29.00/1mg
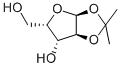
![((3AS,5R,6S,6aS)-6-hydroxy-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)(morpholino)methanone](/CAS/20150408/GIF/1103738-19-7.gif)
1103738-19-7
127 suppliers
$23.00/100mg

1018899-04-1
221 suppliers
$29.00/1mg