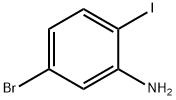
5-BROMO-2-IODOANILINE synthesis
- Product Name:5-BROMO-2-IODOANILINE
- CAS Number:64085-52-5
- Molecular formula:C6H5BrIN
- Molecular Weight:297.92
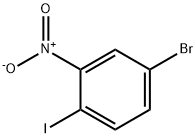
112671-42-8
149 suppliers
$9.00/1g

64085-52-5
124 suppliers
$5.00/250mg
Yield: 93.6%
Reaction Conditions:
with iron;ammonium chloride in tetrahydrofuran;ethanol;water at 70; for 3 h;
Steps:
1.1
A mixture of 4-bromo-1-iodo-2-nitro-benzene (2 g, 6.10 mmol, 1 eq), NH4Cl (1.63 g, 30.50 mmol, 5 eq), Fe (1.70 g, 30.50 mmol, 5 eq) in EtOH (8 mL), THF (8 mL) and H2O (8 mL) was degassed and purged with N2 for 3 times. The mixture was stirred under N2 atmosphere at 70 °C for 3 h. TLC (PE/EtOAc = 5/1, Rf = 0.45) indicated starting material was consumed completely and two new spots formed. The mixture was concentrated and water (80 mL) was added. The mixture was extracted with EtOAc (50 mL x 3) and the combined organic layers were washed with brine (50 mL), dried over Na2SO4, filtered and concentrated to yield a residue which was purified by flash silica gel chromatography (From PE/EtOAc = 1/0 to 5/1, Rf = 0.45) to yield 5-bromo-2- iodo-aniline (1.7 g, 5.71 mmol, 93.6% yield, 100.0% purity) as a light yellow solid.1H NMR (400 MHz, DMSO-d6) d ppm 7.43 (d, J = 8.2 Hz, 1H), 6.89 (d, J = 2.0 Hz, 1H), 6.45 (dd, J = 2.0, 8.2 Hz, 1H), 5.47 (s, 2H); ES-LCMS m/z 298.0, 300.0 [M+H]+.
References:
IKENA ONCOLOGY, INC.;CASTRO, Alfredo C. WO2020/243423, 2020, A1 Location in patent:Paragraph 00465; 00790
875-51-4
300 suppliers
$5.00/1g

64085-52-5
124 suppliers
$5.00/250mg
881-50-5
62 suppliers
$8.00/100mg

64085-52-5
124 suppliers
$5.00/250mg
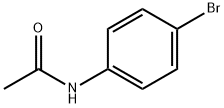
103-88-8
254 suppliers
$6.00/5g

64085-52-5
124 suppliers
$5.00/250mg

88-74-4
5 suppliers
$10.00/1g

64085-52-5
124 suppliers
$5.00/250mg