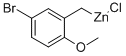
5-BROMO-2-METHOXYBENZYLZINC CHLORIDE synthesis
- Product Name:5-BROMO-2-METHOXYBENZYLZINC CHLORIDE
- CAS Number:352530-35-9
- Molecular formula:C8H8BrClOZn
- Molecular Weight:300.9

352530-35-9
13 suppliers
$228.00/50ml
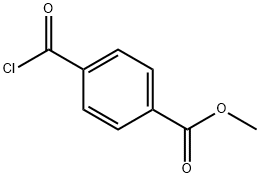
7377-26-6
201 suppliers
$7.00/5g
![Benzoic acid, 4-[2-(5-bromo-2-methoxyphenyl)acetyl]-, methyl ester](/CAS/20210305/GIF/872989-59-8.gif)
872989-59-8
0 suppliers
inquiry
Yield:872989-59-8 69%
Reaction Conditions:
Stage #1: 5-bromo-2-methoxy-benzylzinc chloridewith copper(l) iodide;lithium bromide in tetrahydrofuran at -40 - 55; for 0.416667 h;Cooling with acetone-dry ice;
Stage #2: 4-Methoxycarbonylbenzoyl chloride in tetrahydrofuran at -35 - 20; for 2.5 h;
Steps:
8.Ex-8A
Example 8: 4-[2-(2-Methoxy-5-thien-2-yI-phenyl)-acryloyl]-benzoic acid[0259] Ex-8A: In a 2-neck, 2 L round-bottom flask CuI (30.66 g, 161 mmol) was combined with LiBr (27.97 g, 322 mmol). The flask was placed in a 0 0C bath and THF (360 mL) was added. The mixture exothermed briefly to 55 0C. The solution was cooled in an acetone/CO2 bath and the temperature maintained at -30 to -40 0C while 5-bromo-2- methoxy-benzylzinc chloride (0.46 M in THF, 350 mL, 161 mmol) was added via cannula over 25 min. After addition, the cold bath was removed to allow the solution to warm to -5 0C, then immediately recooled to -30 0C.[0260] In a 500 mL round-bottom flask terephthalic acid monomethyl ester chloride (95%, 35.33 g, 169 mmol) was combined with THF (115 mL). This solution was transferred to the 2 L flask via cannula while maintaining a reaction temperature of -25 to -35 0C. When the transfer was complete, the resulting solution was allowed to warm slowly to room temperature. After 2.5 h, HPLC indicated the reaction was complete. The solution was cooled in a 0 0C bath and 1 N HCl (100 mL) was added slowly in portions. The solution was diluted with H2O (250 mL) and extracted with EtOAc (I x 250 mL, 2 x 100 mL). The organic phase was washed with H2O (4 x 250 mL) and brine (1 x 25 mL), dried over Na2SO4, filtered, and concentrated to ~ 400-500 mL. The resulting mix was stirred rapidly for 25 min, then filtered. The solids were washed with EtOAc (3 x 40 mL) and dried to provide 20.91 g pure product as a beige solid. Additional product was obtained by concentrating the filtrate and subsequently slurrying the resulting solids in EtOAc (2x). A total of 40.1 g (69%) of 4-[2-(5-bromo-2-methoxy- phenyl)-acetyl]-benzoic acid methyl ester was obtained in 3 crops; Crop 3 (4.51 g) contained an impurity. 1H-NMR (CDCl3) δ 8.13 (d, J= 7.8 Hz, 2H), 8.06 (d, J= 7.8 Hz, 2H), 7.36 (dd, J = 7.9, 1.8 Hz, IH), 7.29 (d, J = 1.8 Hz, IH), 6.75 (d, J = 7.9 Hz, IH), 4.25 (s, 2H), 3.96 (s, 3H), 3.75 (s, 3H).
References:
WO2006/4903,2006,A2 Location in patent:Page/Page column 83-84