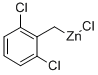
2,6-DICHLOROBENZYLZINC CHLORIDE synthesis
- Product Name:2,6-DICHLOROBENZYLZINC CHLORIDE
- CAS Number:307531-80-2
- Molecular formula:C7H5Cl3Zn
- Molecular Weight:260.86

307531-80-2
23 suppliers
$263.00/50mL
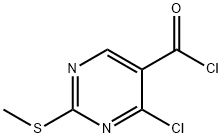
55084-66-7
50 suppliers
$45.00/50mg
![Ethanone, 1-[4-chloro-2-(methylthio)-5-pyrimidinyl]-2-(2,6-dichlorophenyl)-](/CAS/20210305/GIF/1431323-75-9.gif)
1431323-75-9
0 suppliers
inquiry
Yield:1431323-75-9 79%
Reaction Conditions:
Stage #1: (2,6-dichlorobenzyl)zinc(II) chloridewith copper(l) cyanide;lithium chloride in tetrahydrofuran at -25; for 0.25 h;
Stage #2: 4-chloro-2-(methylthio)pyrimidine-5-carbonyl chloride in tetrahydrofuran at -25 - 2; for 1.5 h;Inert atmosphere;
Steps:
1-(4-chloro-2-(methylthio)pyrimidin-5-yl)-2-(2,6-dichlorophenyl)ethanone (8)
Step 1:Preparation of the CuCN·2LiCl solution.To a 250 mL round bottom flask was added copper(I) cyanide (8.95 g, 100 mmol) and lithium chloride (8.48 g, 200 mmol).The flask was capped with septa and heated under high vacuum at 140°C for 3 hours.After cooling to ambient temperature, dry tetrahydrofuran (90 mL) was added and the mixture stirred until the salts were dissolved (24 hours) to give a ~1 Msolution.Step 2: Organozinc addition.To a 1.0 M solution of CuCN:2LiCl (96 mL, 96 mmol) at -25°C was slowly added a 0.5 M solution of (2,6-dichlorobenzyl)zinc(II) chloride (192 mL, 96 mmol).The resulting reaction mixture was stirred for 15 minutes at this temperature.This solution was added over 10 minutes to a stirring solution of the 4-chloro-2-(methylthio)pyrimidine-5-carbonyl chloride (7, 17.85 g, 80 mmol) in 160 mL of dry tetrahydrofuran via cannula.After the addition was complete, the bath was removed and the mixture was stirred at ambient temperature under nitrogen for 1.5 hours.The mixture was quenched with 400 mL of saturated aqueous sodium bicarbonate and after stirring vigourously for 15 minutes, the mixture was extracted with ethyl acetate (3 x 100 mL). The combined organic extracts were washed with brine, dried over magnesium sulfate, filtered and concentrated. The ssolid obtained was recrystalyzed (EtOAc/heaxane) and the filtrate was purified by flash chromatography on a CombiFlash Teledyne Isco system using a Teledyne Isco RediSep Rf gold 220 g silica gel column (eluting 0-30% EtOAc/Hept) to give an additional amount of the title compound. Obtained a total of 21.98 g of final product (79% yield).1H NMR (300 MHz, CDCl3)δppm 8.79 (s, 1H), 7.41 - 7.31 (m, 2H), 7.25 - 7.16 (m,J= 8.8, 7.3, 1H), 4.68 (s, 2H), 2.63 (s, 3H); MS (ESI) m/z 347 (M+H)+.
References:
Mastracchio, Anthony;Lai, Chunqiu;Torrent, Maricel;Bromberg, Kenneth;Buchanan, Fritz G.;Ferguson, Debra;Bontcheva, Velitchka;Johnson, Eric F.;Lasko, Loren;Maag, David;Soni, Nirupama;Shoemaker, Alexander R.;Penning, Thomas D. [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 12,p. 1481 - 1486] Location in patent:supporting information