
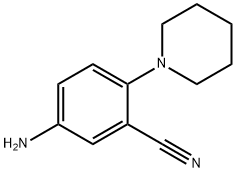
5-BroMo-2-(piperidin-1-yl)benzonitrile synthesis
- Product Name:5-BroMo-2-(piperidin-1-yl)benzonitrile
- CAS Number:876918-30-8
- Molecular formula:C12H13BrN2
- Molecular Weight:265.149

110-89-4
0 suppliers
$15.96/100ML
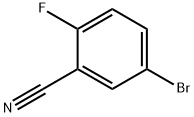
179897-89-3
316 suppliers
$10.00/5g

876918-30-8
12 suppliers
inquiry
Yield:876918-30-8 97%
Reaction Conditions:
Stage #1: 5-Bromo-2-fluoro-benzonitrilewith potassium carbonate in N,N-dimethyl-formamide at 20; for 1 h;
Stage #2: piperidine in N,N-dimethyl-formamide at 80; for 3 h;
Steps:
12.1 Synthesis of 1-(3-cyano-4-piperidinephenyl)imidazole-4-carboxylic acid (A12)
(1) 5-bromo-2-fluorobenzonitrile (1.0 g, 5.0 mmol) was weighed and dissolved in DMF (10 mL), K2CO3 (2.1 g, 15.0 mmol) was added and stirred at room temperature for 1.0 h. Piperidine (1.3 g, 15.0 mmol) was added and the reaction was carried out at 80 ° C for 3 h.TLC followed the reaction completely.Cooled to room temperature, diluted with 100 mL of water, ethyl acetate (100mL × 3). The combined organic phases were washed with brine, dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure,Purified by silica gel column (V ethyl acetate: V petroleum ether = 1:20) to give 5-bromo-2-piperidine-benzonitrile (a12)1.29 g, yield 97%
References:
CN110078668,2019,A Location in patent:Paragraph 0142-0144
34595-33-0
34 suppliers
inquiry

876918-30-8
12 suppliers
inquiry
25016-01-7
233 suppliers
$6.00/5g

876918-30-8
12 suppliers
inquiry
81538-01-4
0 suppliers
inquiry

876918-30-8
12 suppliers
inquiry