
5-BROMO-7-NITRO-1H-INDAZOLE synthesis
- Product Name:5-BROMO-7-NITRO-1H-INDAZOLE
- CAS Number:316810-82-9
- Molecular formula:C7H4BrN3O2
- Molecular Weight:242.03
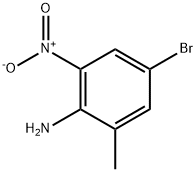
77811-44-0
249 suppliers
$6.00/1g

316810-82-9
67 suppliers
$50.00/100 mg
Yield:316810-82-9 47.7%
Reaction Conditions:
with acetic acid;sodium nitrite in water at 0 - 20; for 6 h;
Steps:
9
Starting material 20a (1.00 g, 4.32 mmol) was dissolved in 15 mL of AcOH.An aqueous solution (2 mL) of sodium nitrite (0.66 mg, 9.52 mmol) was added at 0 °C.After the addition, rt was stirred for 6 hours.The TLC monitors the reaction of the starting material completely, spins the reaction solution, extracts, and the crude product is purified by column chromatography.An orange powder compound 20b (0.5 mg, 2.07 mmol) was obtained in a yield: 47.7%.
References:
Xihua University;Yang Lingling;Qian Shan;Li Guobo;Chen Feng;Li Chao;He Yanying;Wang Zhouyu;Lai Peng CN108689937, 2018, A Location in patent:Paragraph 0179; 0181; 0182
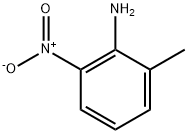
570-24-1
345 suppliers
$6.00/10g

316810-82-9
67 suppliers
$50.00/100 mg