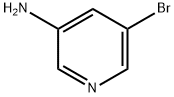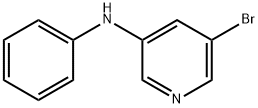
5-Bromo-N-phenylpyridin-3-amine synthesis
- Product Name:5-Bromo-N-phenylpyridin-3-amine
- CAS Number:767342-20-1
- Molecular formula:C11H9BrN2
- Molecular Weight:249.11
Yield:767342-20-1 50%
Reaction Conditions:
with pyridine;oxygen;Cu(OAc)2*H2O;triethylamine in dichloromethane at 40; under 7500.75 Torr; for 2 h;Flow reactor;Green chemistry;Chan-Lam Coupling;
Steps:
A) catalytic Chan-Lam in flow:
General procedure: A solution was prepared from the amine (0.781 mmol) in DCM (5.5 mL) and the boronic acid(1.25 mmol) and NEt3 (0.039 g, 54 μL, 0.391 mmol) were added. A second solution was prepared with Cu(OAc)2*H2O (0.195 mmol, 0.25 equiv), NEt3 (0.039 g, 54 μL, 0.391 mmol) and pyridine (0.062 g, 63 μL, 0.781 mmol) in DCM (5.5 mL). The two solutions were introduced to independent 5 mL sample loop as shown in (Scheme 1). The dispensing HPLC pumps were each set at 0.125 mL/min to achieve a residence time of 2 h. Two reverse “tubein-tube” reactors were used in series to achieve a combined reactor volume of 30 mL which were heated at 40 °C. The reaction mixture was then passed through an Omnifit column (r =0.33 cm, h = 10.00 cm) filled with QP-DMA followed by a back pressure regulator (175 psi).The crude reaction mixture was passed through a plug of silica to remove base line residue and the solvent evaporated under reduced pressure. The resultant crude material was then purified using flash chromatography.
References:
Mallia, Carl J.;Burton, Paul M.;Smith, Alexander M. R.;Walter, Gary C.;Baxendale, Ian R. [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 1598 - 1607] Location in patent:supporting information