
5-Bromoquinoline synthesis
- Product Name:5-Bromoquinoline
- CAS Number:4964-71-0
- Molecular formula:C9H6BrN
- Molecular Weight:208.05

611-34-7
306 suppliers
$6.00/1g

4964-71-0
304 suppliers
$6.00/1g
Yield:4964-71-0 61%
Reaction Conditions:
Stage #1:5-Aminoquinoline with hydrogen bromide;sodium nitrite in water at 0 - 20; for 0.0833333 h;
Stage #2: with hydrogen bromide;copper(I) bromide in water at 20 - 75; for 2 h;
Stage #3: with sodium hydroxide in water
Steps:
7
PREPARATION 7 5-Bromoquinoline Quinolin-5-amine (3.37 g, 23.38 mmol) was dissolved in 9 ml of water and 11 ml of hydrogen bromide (48% in water); the resulting solution was cooled at 0°C. Sodium nitrite (1.94 g, 28.12 mmol) dissolved in 9 ml of water was dropwise added. The resultant solution was stirred at room temperature for 5 minutes. This solution was dropwise added to a solution of copper(I) bromide (4.02 g, 28.02 mmol) in 23 mL of HBr (48% in water) at 75°C. The resulting mixture was stirred at room temperature for 2 h. Then the reaction mixture was basified with sodium hydroxide and extracted twice with ethyl acetate. The organic phase is washed with brine, filtered and dried over sodium sulfate. After filtration and evaporation of the solvent, 2.98 g (61 %) of the final product were obtained. 1H NMR (CDCl3) δ ppm: 7.44 - 7.63 (m, 2 H) 7.84 (d, J=7.42 Hz, 1 H) 8.10 (d, J=8.24 Hz, 1 H) 8.56 (d, J=8.51 Hz, 1 H) 8.94 (br.s., 1H) HPLC/MS (9 min) retention time 5.68 min. LRMS: m/z 208 (M)/21 0 (M+2)
References:
Almirall, S.A. EP2394998, 2011, A1 Location in patent:Page/Page column 16
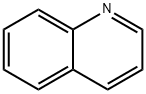
91-22-5
397 suppliers
$10.00/25g

4964-71-0
304 suppliers
$6.00/1g

846038-45-7
12 suppliers
inquiry

4964-71-0
304 suppliers
$6.00/1g
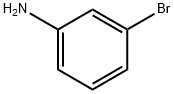
591-19-5
326 suppliers
$10.00/10g

4964-71-0
304 suppliers
$6.00/1g

89598-96-9
350 suppliers
$9.00/1g

4964-71-0
304 suppliers
$6.00/1g