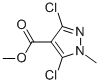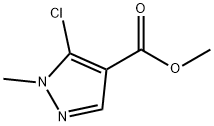
5-Chloro-1-methyl-1H-pyrazole-4-carboxylic acid synthesis
- Product Name:5-Chloro-1-methyl-1H-pyrazole-4-carboxylic acid
- CAS Number:88398-85-0
- Molecular formula:C6H7ClN2O2
- Molecular Weight:174.59
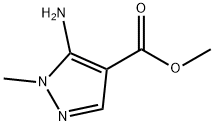
110860-60-1
34 suppliers
$44.00/250mg

88398-85-0
21 suppliers
inquiry
Yield:88398-85-0 82.3%
Reaction Conditions:
with hydrogenchloride;phosphoric acid;sulphurous acid;acetic acid;sodium nitrite;copper(I) chloride in water;
Steps:
R.4 Methyl 5-chloro-1-methylpyrazole-4-carboxylate
REFERENCE EXAMPLE 4 Methyl 5-chloro-1-methylpyrazole-4-carboxylate 12.1 g of methyl 5-amino-1-methylpyrazole-4-carboxylate is dissolved in a mixture of 28 ml of concentrated hydrochloric acid, 18 ml of acetic acid and 15.6 ml of phosphoric acid. While cooling at -5° C. to -7° C. and stirring, a solution of 5.5 g of sodium nitrite in 7 ml of water is added dropwise to the mixture over a period of 35 minutes to prepare the diazonium salt solution. Separately, to 95 ml of acetic acid saturated with sulfurous acid gas at 0° C. to 3° C., is added 1.6 g of cuprous chloride. To this solution is added dropwise the diazonium salt solution prepared previously over a period of 30 minutes. The reaction mixture is stirred at 0° C. to 4° C. for 2 hours, and then the reaction mixture is poured into 500 ml of water, and extracted with ether. The extract is washed with a saturated aqueous sodium chloride solution, a saturated aqueous sodium bicarbonate solution and a saturated aqueous sodium chloride solution in that order, and dried over anhydrous sodium sulfate. Ether is distilled off to yield 11.6 g of a yellow oil. After the oil is dissolved in hot water, the solution is cooled. Colorless needle crystals separate. The crystals are filtered and dried to yield 11.2 g of the title compound Yield 82.3%. mp. 69 to 70° C. IR ν (nujol) cm-1: 1720, 1540, 1227, 1042, 772
References:
US4872901,1989,A

110860-60-1
34 suppliers
$44.00/250mg

88398-85-0
21 suppliers
inquiry
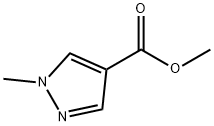
5952-93-2
64 suppliers
$60.00/100mg