
5-cyano-1-methyl-1H-pyrrol-2-ylboronic acid synthesis
- Product Name:5-cyano-1-methyl-1H-pyrrol-2-ylboronic acid
- CAS Number:860617-71-6
- Molecular formula:C6H7BN2O2
- Molecular Weight:149.94
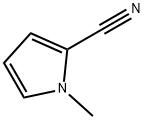
34884-10-1
88 suppliers
$45.95/250mgs:

860617-71-6
30 suppliers
inquiry
Yield: 70%
Reaction Conditions:
Stage #1:1-methyl-1H-pyrrole-2-carbonitrile with Triisopropyl borate;lithium diisopropyl amide in tetrahydrofuran at -4 - 13; for 2 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran
Steps:
1.C
The solution of 1-methylpyrrole-2-carbonitrile (0.131 g, 1.23 mmol), triisopropylborate (250 µL, 1.08 mmol), and THF (4 mL) was cooled to about -4 °C and 2M LDA (0.6 mL, 1.2 mmol) was added dropwise from a syringe. The bath was removed and the suspension was allowed to warm to about 13 °C within about 2 hours. After cooling and quenching with water and 5% aqueous HCI, the product was extracted with diethylether. The organic layer was evaporated to give an oil which solidified upon standing to give 0.130 g (70% yield) of (5-cyano-1-methyl-1H-pyrrol- 2-yl) boronic acid. ¹H-NMR (DMSO-d6) : No. 8.38, 6.87, 6.77, and 3.88.
References:
WYETH WO2005/105817, 2005, A2 Location in patent:Page/Page column 24
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

860617-71-6
30 suppliers
inquiry
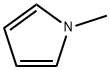
96-54-8
273 suppliers
$5.00/1g

860617-71-6
30 suppliers
inquiry