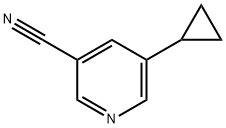
5-cyclopropylnicotinonitrile synthesis
- Product Name:5-cyclopropylnicotinonitrile
- CAS Number:900802-81-5
- Molecular formula:C9H8N2
- Molecular Weight:144.17
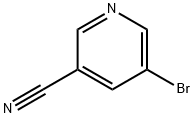
35590-37-5
225 suppliers
$6.00/250mg
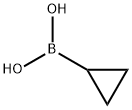
411235-57-9
425 suppliers
$6.00/1g

900802-81-5
12 suppliers
inquiry
Yield:900802-81-5 58%
Reaction Conditions:
with dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;potassium phosphate;palladium diacetate in tetrahydrofuran; for 16 h;Inert atmosphere;Reflux;
Steps:
1 Step 1 : 5-Cyclopropylnicotinonitrile
Step 1 : 5-Cyclopropylnicotinonitrile To a solution of 3-bromo-5-cyanopyridine (1 g, 5.3 mmol) in tetrahydrofuran was added cyclopropyl boronic acid (0.5 g, 5.6 mmol) and potassium phosphate (1.08 g, 15.9 mmol). The resulting solution was degassed with nitrogen for 10 minutes and then palladium (II) acetate and S-phos were added. The reaction mixture was refluxed for 16 h. The reaction mixture was diluted with water, extracted with ethyl acetate and concentrated under reduced pressure. The crude material was purified via column chromatography to afford 5-cyclopropylnicotinonitrile (0.45 g, 58%). MS (ES+) (M+H) 145.1774..
References:
WO2013/150416,2013,A1 Location in patent:Page/Page column 99

35590-37-5
225 suppliers
$6.00/250mg
23719-80-4
170 suppliers
$85.20/25ml

900802-81-5
12 suppliers
inquiry

35590-37-5
225 suppliers
$6.00/250mg
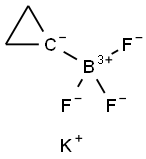
1065010-87-8
152 suppliers
$6.00/250mg

900802-81-5
12 suppliers
inquiry