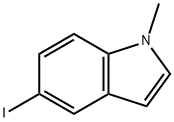
5-iodo-1-methylindoline-2,3-dione synthesis
- Product Name:5-iodo-1-methylindoline-2,3-dione
- CAS Number:76034-84-9
- Molecular formula:C9H6INO2
- Molecular Weight:287.05

20780-76-1
149 suppliers
$6.00/250mg

74-88-4
342 suppliers
$15.00/10g

76034-84-9
8 suppliers
inquiry
Yield: 100%
Reaction Conditions:
Stage #1:5-iodoisatin with sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.0833333 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;
Steps:
3-Hydroxy-5-iodo- h-methyl-3-phenylindolin-2-one (1 hh).
5-lodoisatin (8a) (1.0 g, 3.7 mmol) was dissolved in anhydrous DMF (15 mL) and cooled to0 °C, whereupon NaH (60% dispersion in mineral oil; 175 mg; 4.4 mmol) was added in one portion and the resulting red slurry stirred for 5 minutes. lodomethane (0.34 mL; 5.5 mmol) was added and the reaction was stirred at 0 °C for 30 minutes. The mixture was then poured into aqueous saturated NH4C1 (30 mL) and extracted with EtOAc (3 x 20 mL). The combined organic layers were washed with water (15 mL) and brine (20 mL), dried (MgSO4), filtered and concentrated to give crude N-methyl-5-iodoisatin (8b) (100%) which was used without further purification.
References:
LATVIAN INSTITUTE OF ORGANIC SYNTHESIS WO2021/130515, 2021, A1 Location in patent:Paragraph 041; 050
280563-07-7
10 suppliers
inquiry

76034-84-9
8 suppliers
inquiry
![Ethanone, 1-[2-(methylamino)phenyl]- (9CI)](/CAS/GIF/1859-75-2.gif)
1859-75-2
29 suppliers
$72.00/100mg

76034-84-9
8 suppliers
inquiry
219605-52-4
0 suppliers
inquiry

76034-84-9
8 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)