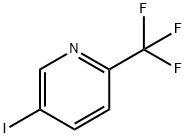
5-IODO-2-(TRIFLUOROMETHYL)PYRIDINE synthesis
- Product Name:5-IODO-2-(TRIFLUOROMETHYL)PYRIDINE
- CAS Number:873107-98-3
- Molecular formula:C6H3F3IN
- Molecular Weight:272.99
106877-33-2
275 suppliers
$8.00/1g

873107-98-3
70 suppliers
$60.00/100mg
Yield:873107-98-3 87%
Reaction Conditions:
Stage #1: 6-(trifluoromethyl)pyridin-3-aminewith hydrogenchloride;sodium nitrite in water at -5 - 5; for 0.166667 h;
Stage #2: with potassium iodide in water at -5 - 10;
Steps:
1.142.1 Step 1: 5-iodo-2-(trifluoromethyl)pyridine [0251] A solution of 6-(trifluoromethyl)pyridin-3-amine
Step 1: 5-iodo-2-(trifluoromethyl)pyridine [0251] A solution of 6-(trifluoromethyl)pyridin-3-amine (9.96 g, 0.062 mol) in 5N HC1 (70 mL) was cooled to -5°C and sodium nitrite (6.39 g, 0.093 mol) in 30 mL of water was added dropwise while maintaining the internal temperature below 5°C. After 10 min, KI (22.5 g, 0.136 mol) in 30 mL of water was added dropwise at -5°C while maintaining the internal temperature below 10°C over the course of the addition. The reaction mixture was warmed to rt and 250 mL of EtOAc was added. The pH of the aqueous layer was adjusted to 11 by the addition of 50 mL of 6N NaOH, the layers were separated, and the organic layer was washed with 120 mL of 0.3M Na2S203. The EtOAc layer was concentrated and the concentrate was purified by column chromatography over silica gel (hexane/ EtOAc =25/1) to afford the title compound as a white solid (14.6 g, 87%). MS (ESI) calcd for C6H3F3IN: 273.0; found: 274.0[M+H]. 1H NMR (400 MHz, CDC13) δ 8.96 (s, 1H), 8.22 (d, J = 8.2 Hz, 1H), 7.47 (d, J = 8.2 Hz, 1H).
References:
WO2015/187845,2015,A1 Location in patent:Paragraph 0251
436799-32-5
329 suppliers
$5.00/250mg

873107-98-3
70 suppliers
$60.00/100mg