
5-Methoxybenzofuran-2-carbaldehyde synthesis
- Product Name:5-Methoxybenzofuran-2-carbaldehyde
- CAS Number:23145-19-9
- Molecular formula:C10H8O3
- Molecular Weight:176.17
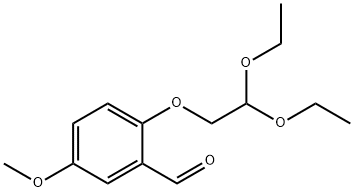
1254062-17-3
1 suppliers
inquiry

23145-19-9
30 suppliers
$45.00/10mg
Yield:23145-19-9 70%
Reaction Conditions:
with acetic acid for 24 h;Reflux;
Steps:
General procedures for the synthesis of substitutedBenzo[b]furan-2-yl carboxaldehydes 4a-g: Method C
General procedure: A stirred solution of compounds 3 (0.1 mol) in 35 mL ofconcentrated acetic acid was refluxed for 24 h. After cooling,the solution was evaporated to dryness. The crude productwas distilled or recrystallized from an appropriate solvent.
References:
Boukharsa, Youness;Meddah, Bouchra;Tiendrebeogo, Ramata Yvette;Ibrahimi, Azeddine;Taoufik, Jamal;Cherrah, Yahia;Benomar, Ali;Faouzi, My El Abbes;Ansar, M'Hammed [Medicinal Chemistry Research,2016,vol. 25,# 3,p. 494 - 500]
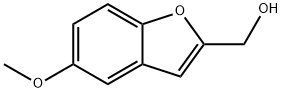
37603-26-2
10 suppliers
inquiry

23145-19-9
30 suppliers
$45.00/10mg
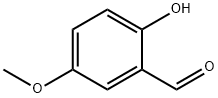
672-13-9
265 suppliers
$39.00/5g

23145-19-9
30 suppliers
$45.00/10mg

10242-08-7
114 suppliers
$18.00/250mg

23145-19-9
30 suppliers
$45.00/10mg
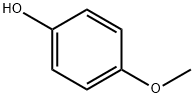
150-76-5
754 suppliers
$5.00/5G

23145-19-9
30 suppliers
$45.00/10mg