
5-METHYL-3-NITROMETHYL-HEXANOIC ACID synthesis
- Product Name:5-METHYL-3-NITROMETHYL-HEXANOIC ACID
- CAS Number:181289-21-4
- Molecular formula:C8H15NO4
- Molecular Weight:189.21
![Propanedioic acid, 2-[3-methyl-1-(nitromethyl)butyl]-, 1,3-dimethyl ester](/CAS/20200611/GIF/100401-40-9.gif)
100401-40-9
0 suppliers
inquiry

181289-21-4
14 suppliers
inquiry
Yield:181289-21-4 94.8%
Reaction Conditions:
with methanesulfonic acid;water;acetic acid at 110; for 6 h;Concentration;Reagent/catalyst;Temperature;Time;
Steps:
1.31 Optimization of One-Pot Hydrolysis and Decarboxylation of Dimethyl 2- (4-methyl-l- nitropentan-2-yl) malonate (XVI)
Due to previous non-satisfying results (entries 1-2), the one-pot hydrolysis/decarboxylation was investigated extensively. Optimization of the reaction started from HC1 in water (entry 3) as described in WO 2009/147434. To reduce the formation of XVII, the amount of water was decreased by using AcOH as solvent instead, and also the amount of HC1 was reduced (entry 4, 5) . A slight increase of the amount of HC1 from 6 to 8 did not improve the result. The amount of water was further decreased by using more concentrated HC1 (entries 6 and 7), which improved the ratio of IX:XVII, but incomplete reaction was observed in entry 7 (HPLC: 44% of product with 10% of XVI) . Increasing the reaction temperature to 110 °C significantly accelerated the reaction, although the concentration and water amount were lowered (comparing entry 8 with entries 6-7) . The reaction rate comes to limit and low conversion (HPLC: 5% of product) was observed when only 2.5 equiv. of HC1 were used (entry 9) . To investigate the influence of acid amount without variation of the amount of water, MsOH was used as the strong acid S. Starting from 2.5 equiv., the ratio of IX: XVI I was improved but about 10% of imide XVIII was also observed (entries 10 and 11) . Although the reaction with 10 equiv. of water was carried out at 110 °C, less than 10% of XVII was obtained. There was no by-product formation at 90 °C in the presence of various amounts of MsOH and water (entries 12, 13, and 14), however longer reaction time (1 d) was needed. Under these conditions (90 °C, 8 equiv. of water, 0.5 M) , XVII can be observed when using 4-6 equiv. of MsOH (comparing entry 14 with entries 18 and 19) . Even though the reaction with a lower amount of MsOH (0.5-1.0 equiv.) was performed at a higher temperature (110 °C) , still no XVII was formed (comparing entry 14 with entries 15 and 16) . It comes to limit when the reaction is carried out with 2 equiv. of MsOH at 110 °C (comparing entry 15 with 17), where XVII was formed. Apparently, the amount of acid (0.5-2 equiv.) and temperature influence to the reaction rate: the higher the faster (entries 15-17 for acid amount and entries 11-12 for temperature) . Using more than 3.5 equiv. of MsOH (entries 18-19) did not change the reaction rate significantly. The desired product can be obtained in >80% yield with 0.5-4 equiv. of MsOH (entries 15-18). The initial optimization shows that the formation of XVII and XVIII can be favorably suppressed. In order to get a more robust procedure, Taguchi experiment (L9 array; see table 2) was performed (entries 20-28) . Four factors (acid amount (A), concentration (C) , water amount (W) , and temperature (T) ) , each at 3 levels were considered. Table 2: For most cases, the reaction gave high yield (>83%) , except entry 24. For entries 25 and 28, low yield was obtained because of incomplete reaction (15% and 13%, respectively, as determined by 1R NMR) . As shown in entries 20, 25, and 27, long reaction time is needed at 90 °C. XVII is always formed if the reaction is conducted at high concentration (1.0 M; entries 22, 23, and 27) and is never observed at low concentration (0.5 M; entries 20, 24, and 28) . The best yield was obtained in entry 21, where 2.5 equiv. of MsOH were used. After performing statistical analysis of these results, the following significant process parameters were identified: - Factors influencing the reaction rate: T>OW>A - Factors influencing the yield: A>W>OT - Factors influencing the amount of XVII: OA>W>T Consequently, reaction with MsOH (2.5 equiv.), water (8 equiv.) at 0.5 M and 110 °C should provide the highest yield . A confirmation experiment (entries 29 with racemic substrate and 30 with enantiomerically enriched substrate) was conducted to verify the optimal process parameters obtained from the process parameter design. Scaling up economically by using crude Michael adduct under the same conditions (entries 31-32) gave product IX in about 70% yield over 2 steps (Michael addition and hydrolysis/dacarboxylation) . Higher concentration showed again more by-product formation (entry 31) . To get more cost effective, MsOH was replaced by H2 S O4 (entries 33-43) . The by-products were exclusively formed if water amount was very low (1.2 equiv., entry 33) . Using the best condition found for MsOH with H2 S O4 (entry 34), 7% of XVII was formed. To evaluate the factors (A, W, and T) , design of experiment was again performed (entries 36-39) . The best conditions (1.2 equiv. of H2 S O4 , 8 equiv. of water at 100 or 110 °C, entries 40 and 41) were established by statistical analysis. This afforded the product IX in more than 80% yield over 2 steps (Michael addition and hydrolysis/dacarboxylation), which was higher than for MsOH (comparing entry 41 with entry 32) . When using less H2 S O4 (0.4 equiv.), the reaction time increased (entry 42), but almost the same result was obtained (comparing entry 41 with 42). Using recovered AcOH (-5.8% water contained), the reaction successfully took place to afford product IX in up to 94% HPLC yield (entry 43) . In conclusion, it was found that MsOH (0.5-6.0 equiv.) and H2S04 (0.4-2.5 equiv.) can be used to perform the one-pot hydrolysis and decarboxylation reaction of XVI successfully. However, a significant amount of water (6- 10 equiv.) is necessary to get gratifying results. - 2 The reaction rate and the product ratio were mainly influenced by the reaction temperature (110 °C) and the concentration (0.5 M) . When applying this robust procedure, exclusively the desired product IX was formed in up to 94% yield for one step (using pure XVI) or 70-87% yield over two steps (using crude XVI) .
References:
WO2015/189068,2015,A1 Location in patent:Page/Page column 24-28; 42
![Isoxazole, 3-methyl-5-[4-methyl-2-(nitromethyl)pentyl]-4-nitro-](/CAS/20210305/GIF/1439214-11-5.gif)
1439214-11-5
0 suppliers
inquiry

181289-21-4
14 suppliers
inquiry
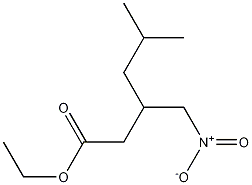
128013-65-0
19 suppliers
inquiry

181289-21-4
14 suppliers
inquiry

590-86-3
380 suppliers
$10.00/5g

181289-21-4
14 suppliers
inquiry
