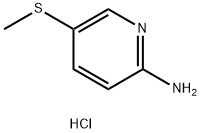
5-(Methylthio)pyridin-2-aMine Hydrochloride synthesis
- Product Name:5-(Methylthio)pyridin-2-aMine Hydrochloride
- CAS Number:909711-79-1
- Molecular formula:C6H9ClN2S
- Molecular Weight:176.66

20511-12-0
459 suppliers
$6.00/1g
5188-07-8
268 suppliers
$13.00/5g

909711-79-1
4 suppliers
$650.00/500mg
Yield:909711-79-1 86%
Reaction Conditions:
Stage #1: 2-amino-5-iodopyridine;sodium thiomethoxidewith potassium carbonate;ethylene glycol;copper(l) iodide in isopropyl alcohol at 80; for 24 h;
Stage #2: with hydrogenchloride in 1,4-dioxane;
Steps:
7
To a mixture of 5-iodopyridin-2-amine (2.Og, 9.09mmol), anhydrous potassium carbonate (2.5g, 18.18mmol), sodium methanethiolate (1.27g, 18.18mmol) and cuprous iodide (172mg, 0.909mmol) in isopropanol (27ml), ethylene glycol (1.01ml, 18.18mmol) was added. The reaction was stirred at 80 0C under nitrogen for 24 hours. The reaction mixture was diluted with EtOAc and washed in turn with water (twice), saturated sodium chloride, dried with anhydrous sodium sulphate, filtered and evaporated. The residue was dissolved in ether and filtered to remove an insoluble impurity. The filtrate was taken and treated with excess hydrogen chloride in 1 ,4-dioxane. The precipitated solid was filtered, washed with ether and dried to give the title compound as a white solid (1.385g, 86%). NMR: 2.43 (s, 3H), 7.0 (d, IH), 7.9 (m, 2H); m/z 140.
References:
WO2006/95159,2006,A1 Location in patent:Page/Page column 62